Modern breast surgery: What you should know
In this Article
- Counseling patients on CPM
- Oncoplastic lumpectomy approach
- How to manage the lymph nodes
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.
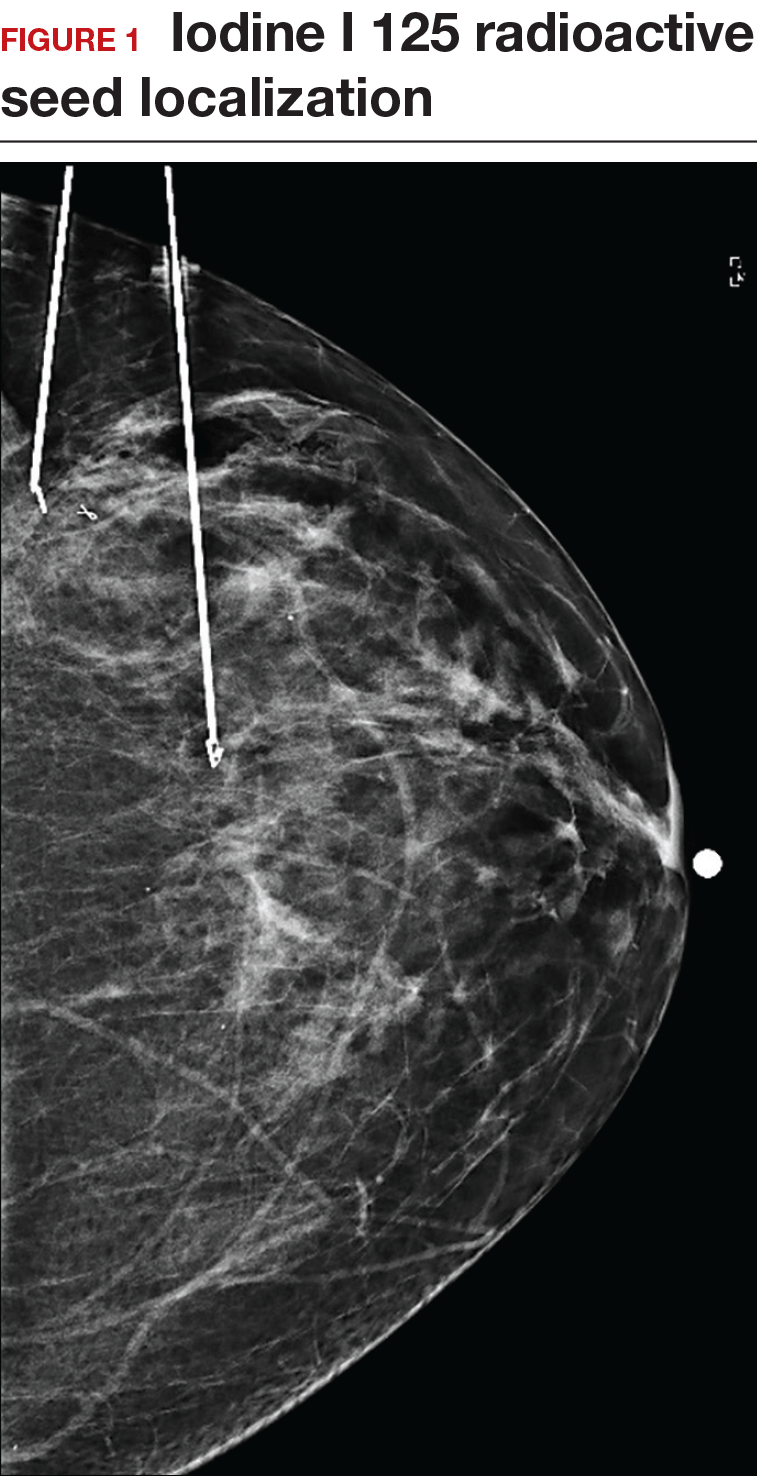
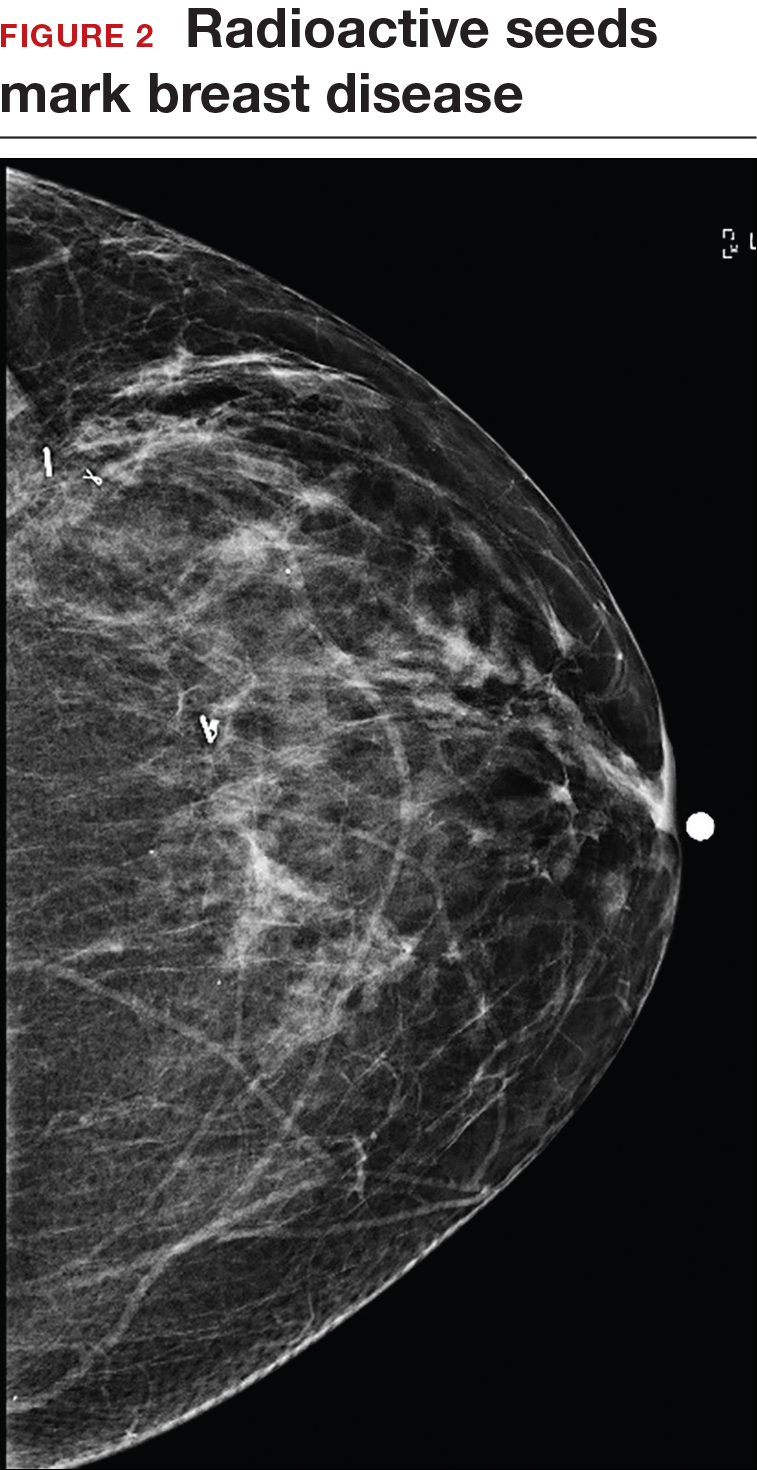
Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.
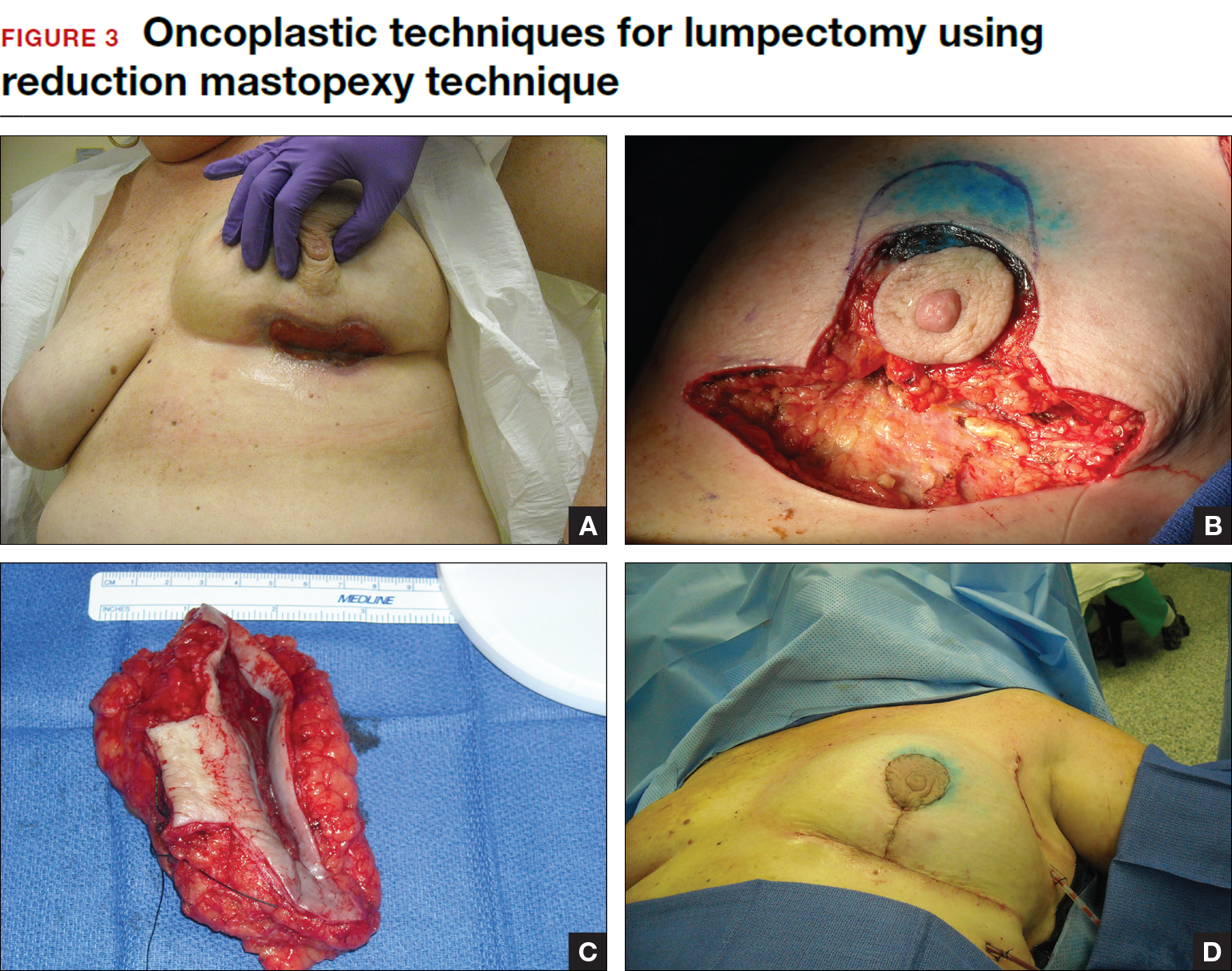
Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.
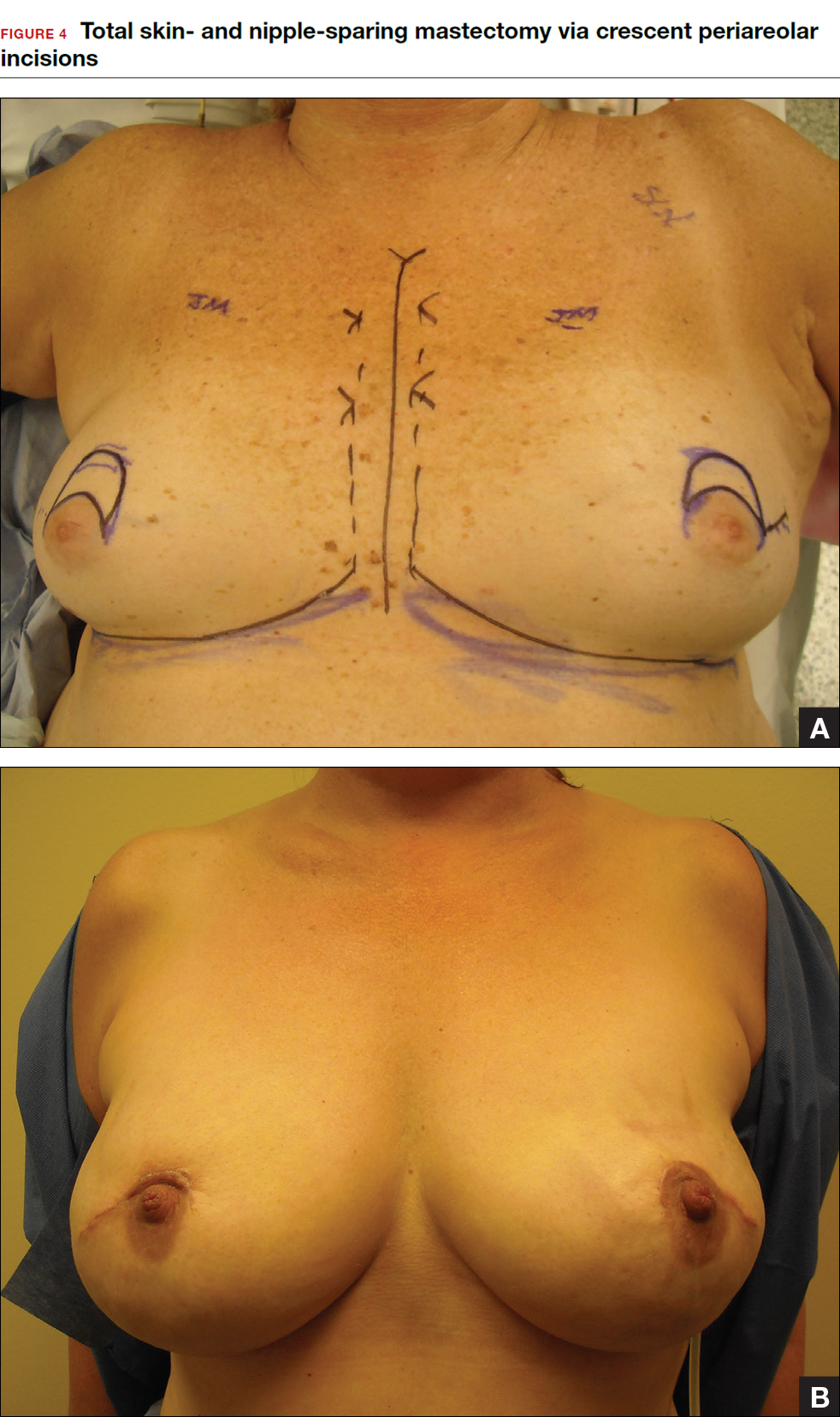
Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12







