Your teenage patient and contraception: Think “long-acting” first
Although use of long-acting reversible contraception is increasing slowly in the United States, there is plenty of room for improvement, particularly among young women. Here, 2 experts address the nuances of choosing a method for a teenage patient.
In this Article
- A comparative look at 5 LARC methods
- Common barriers to LARC
- How to insert Liletta
Noncontraceptive benefits include reduced bleeding
The 3 LNG-IUS methods and the subdermal implant offer several benefits beyond contraception. Because of their progestin content, these methods reduce or even eliminate menses. This benefit can be very helpful for women who experience heavy menstrual periods and the consequent risk of anemia. Because of reduced menstrual flow, users of hormonal LARC methods also commonly experience less cramping associated with menses.
Women with endometriosis often benefit from hormonal LARC methods, as the disease is suppressed by the progestin component. Users of IUDs also have a reduced risk of endometrial cancer.
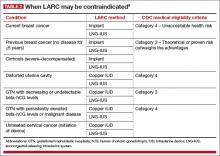
Contraindications to LARC
There are few contraindications to LARC methods, making them an appropriate choice for most women. The US Medical Eligibility Criteria for Contraceptive Use, 2010, published by the Centers for Disease Control and Prevention (CDC), contain guidelines that are based on the best available evidence.9 Contraceptive methods that are labeled as Category 1 or 2 are not contraindicated for most women. Methods that fall into Category 3 (theoretical or proven risks outweigh the advantages) or Category 4 (unacceptable health risk) are contraindicated (TABLE 2).9
IUDs once were thought to expose women to an increased risk of pelvic inflammatory disease, but this fear has long been disproven. Screening for chlamydia can be performed at the time of placement, as recommended annually for women younger than 25 years. Unless there is concern for active cervical or uterine infection, there is no need to delay insertion of an IUD while awaiting test results. In most cases, women found to have positive cultures after insertion can be treated successfully without IUD removal.
Main adverse effect is altered bleeding patterns
Adverse effects vary depending on the method being used. All LARC methods may affect menstrual patterns. For example, clinical trials involving the copper IUD indicate that abnormal heavy bleeding may lead to discontinuation in up to 10% of users.5,10 Amenorrhea or oligomenorrhea is uncommon with this method and rarely leads to discontinuation. For example, in one trial involving more than 900 women using a copper IUD for up to 5 years, there were no discontinuations due to amenorrhea. Dysmenorrhea may arise, but data from clinical trials indicate that its frequency decreases over time. In one trial, the frequency of any menstrual pain decreased from about 9% of users to 5% after 8 months or more of use.
The LNG-IUS also can be associated with abnormal uterine bleeding. In contrast to the copper IUD, LNG devices tend to reduce menstrual bleeding and can be unpredictable. Clinical trials involving the 5-year 52-mg LNG-IUS indicate that bleeding decreases over time, with as many as 70% of users developing amenorrhea or oligomenorrhea.5,11 However, some women using an LNG-IUS experience heavy bleeding— although the frequency of such bleeding tends to be substantially less than that experienced by copper IUD users.7
A lack of comparative trials makes it unclear whether the newer 3-year LNG-IUS devices are associated with a significantly altered bleeding pattern. Noncomparative data from the package insert for Skyla suggest that women using it may have a higher frequency of heavy menstrual bleeding and less amenorrhea than users of the 5-year device.6
Data from a 3-year clinical trial of the newest 52-mg LNG-IUS (Liletta) indicate that bleeding and dysmenorrhea led to discontinuation 1% to 2% of the time.2
Although the concentration of progestincirculating systemically is low with the various LNG-IUS devices, some women may experience symptoms such as mood swings, headaches, acne, and breast tenderness.
Expulsions during the first year of use of the copper IUD and the 3 LNG-IUS devices range from 2% to 10%, with the higher rates associated with immediate postpartum insertion.5
Uterine perforation has been reported in about 1 of every 1,000 insertions. Other adverse events are uncommon.
Clinical trials indicate that about 11% of implant users will discontinue the method due to bleeding abnormalities.12 About 25% to 30% of users will experience heavy or prolonged bleeding, while up to 33% will experience infrequent bleeding or amenorrhea. About 50% of implant users will experience improved bleeding patterns over time.
Other reasons for discontinuation of implant use in a very small percentage of users include emotional lability, weight gain, acne, and headaches.4 Complications due to insertion and removal are rare and include pain, bleeding, and hematoma formation.
Public health impact of LARC methods
An important question in regard to LARC use is: How do we best provide safe and effective contraception for teens and young adult women? There is increasing evidence that, with appropriate counseling and the removal of cost barriers, LARC methods can have a significant public health impact in this population.