Get ready for a practice makeover
HPV vaccine imminent … Colposcopy disappoints … LEEP raises preterm risk … LSIL meaningless?
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
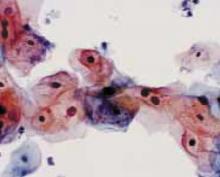
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.