The Effects of a Multifaceted Intervention to Improve Care Transitions Within an Accountable Care Organization: Results of a Stepped-Wedge Cluster-Randomized Trial

This work is licensed under a Creative Commons Attribution 4.0 International License
BACKGROUND: Transitions from hospital to the ambulatory setting are high risk for patients in terms of adverse events, poor clinical outcomes, and readmission.
OBJECTIVES: To develop, implement, and refine a multifaceted care transitions intervention and evaluate its effects on postdischarge adverse events.
DESIGN, SETTING, AND PARTICIPANTS: Two-arm, single-blind (blinded outcomes assessor), stepped-wedge, cluster-randomized clinical trial. Participants were 1,679 adult patients who belonged to one of 17 primary care practices and were admitted to a medical or surgical service at either of two participating hospitals within a pioneer accountable care organization (ACO).
INTERVENTIONS: Multicomponent intervention in the 30 days following hospitalization, including: inpatient pharmacist-led medication reconciliation, coordination of care between an inpatient “discharge advocate” and a primary care “responsible outpatient clinician,” postdischarge phone calls, and postdischarge primary care visit.
MAIN OUTCOMES AND MEASURES: The primary outcome was rate of postdischarge adverse events, as assessed by a 30-day postdischarge phone call and medical record review and adjudicated by two blinded physician reviewers. Secondary outcomes included preventable adverse events, new or worsening symptoms after discharge, and 30-day nonelective hospital readmission.
RESULTS: Among patients included in the study, 692 were assigned to usual care and 987 to the intervention. Patients in the intervention arm had a 45% relative reduction in postdischarge adverse events (18 vs 23 events per 100 patients; adjusted incidence rate ratio, 0.55; 95% CI, 0.35-0.84). Significant reductions were also seen in preventable adverse events and in new or worsening symptoms, but there was no difference in readmission rates.
CONCLUSION: A multifaceted intervention was associated with a significant reduction in postdischarge adverse events but no difference in 30-day readmission rates.
© 2021 Society of Hospital Medicine
To evaluate the effects of the intervention on nonelective readmissions and preventable readmissions, we used a similar approach, using multivariable logistic regression. Postdischarge functional status, adjusted for status prior to admission, was analyzed using multivariable linear regression and random effects by primary care practice. The general linear mixed model (GLIMMIX) procedure in the SAS 9.3 statistical package (SAS Institute) was used to carry out all analyses.
Power and Sample Size
We assumed a baseline rate of postdischarge adverse events of 0.30 per patient.21 We conservatively assumed an effect size of a change from 0.30 in the control group to 0.23 in the intervention group (a relative reduction of 22%, which was based on studies of preventability rates23 and close to the minimum clinically important difference). Based on prior studies,4,22 we assumed an intraclass correlation coefficient of 0.01 with an average cluster size of seven patients per PCP. Assuming a 10% loss to follow-up rate and an alpha of 0.05, we targeted a sample size of 1,800 patients to achieve 80% power, with one-third of the patients in the usual care arm and two-thirds in the intervention arm.
RESULTS
We enrolled 18 PCMH primary care practices to participate in the study, including 8 from Hospital A (out of 13 approached), 8 from Hospital B (out of 11), and 2 from other ACO practices (out of 9) (plus two pilot practices). Reasons for not participating included not having dedicated personnel to play the role of the responsible outpatient clinician, undergoing recent turn-over in practice leadership, and not having enough patients admitted to the two hospitals. One practice only enrolled 5 patients in the study and withdrew from participation, which left 17 practices.
Study Patients
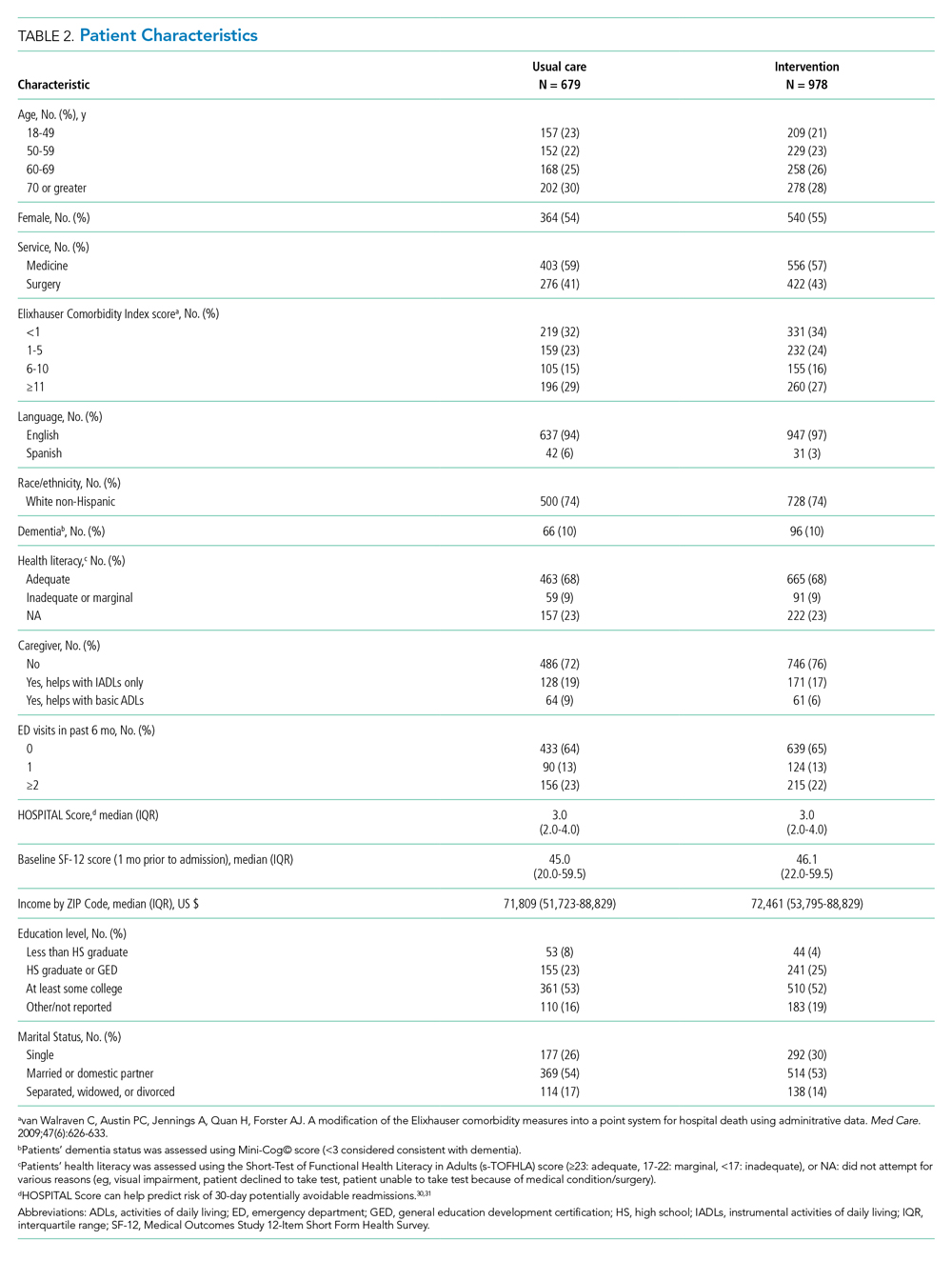
We enrolled 1,679 patients (Figure 1). Reasons for nonenrollment included being unable to complete the screen prior to discharge, not meeting inclusion criteria or meeting exclusion criteria, being assigned to a pilot practice, and declining informed written consent. The baseline characteristics of enrolled patients are presented in Table 2. Differences between the two study arms were small. About 47% of the cohort was not reachable by phone after five attempts for the 30-day phone call, but only 69 (4.1%) were truly lost to follow-up because they were unreachable by phone and had no documentation in the electronic medical record in the 30-days after discharge.
Intervention Fidelity
The majority of patients did not receive most intervention components, even those components that were supposed to be delivered to all intervention patients (Table 3). A minority of patients were referred to visiting nurse services and to the home pharmacy program. However, 855 patients (87%) in the intervention arm received at least one intervention component.

Outcome Measures
The intervention was associated with a statistically significant reduction in several of the outcomes of interest, including the primary outcome, number of postdischarge adverse events (45% reduction), and new or worsening postdischarge signs or symptoms (22% reduction), as well as preventable postdischarge adverse events (58% reduction) (Table 4). There was a nonsignificant difference in functional status. There was no significant effect on total nonelective or on preventable readmission rates. When analyzed by type of adverse event, the intervention was associated with a reduction in adverse drug events and in procedural complications (Table 2 of Appendix 1). Of note, there was no significant difference in the proportion of patients with at least one adverse event whether the outcome was determined by phone call and medical record review (49%) or medical record review alone (51%) (P = .48).
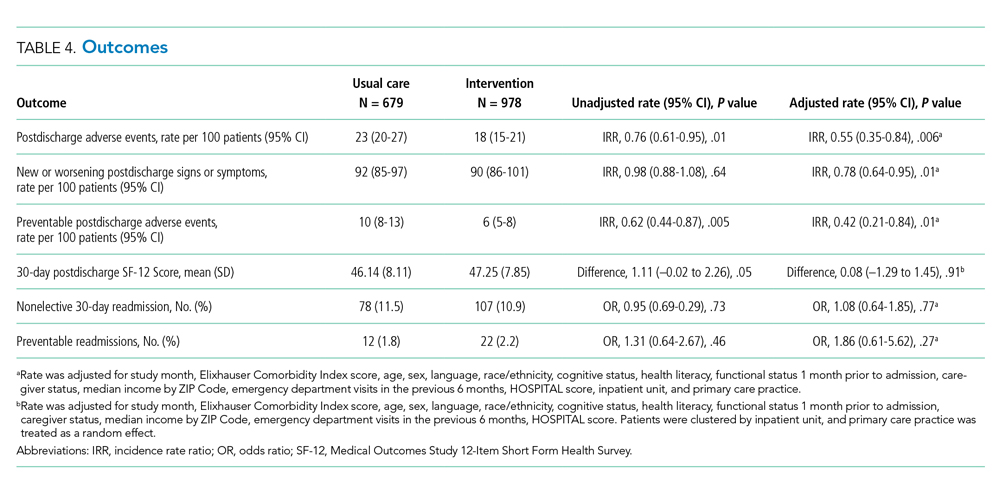
In subgroup analyses, there was no evidence of effect modification by service, hospital, patient age, readmission risk, health literacy, or comorbidity score (Table 3 of Appendix 1). Table 4 of Appendix 1 provides examples of postdischarge adverse events seen in the usual care arm that might have been prevented in the intervention.






