Small-Cell Lung Cancer
Role of Surgery
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging [29,30]. Most of the data in this setting are derived from retrospective studies [31,32]. A 5-year OS of 40% to 60% has been has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if no nodal involvement) or chemoradiation (if nodal involvement) is recommended [33,34]. Wedge or segmental resections are not considered to be optimum surgical options.
Case Continued
The patient received 4 cycles of cisplatin and etoposide along with 70 Gy radiation concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans showed partial response (PR). The patient underwent PCI 6 weeks after completion of treatment. Eighteen months later, the patient comes to the clinic for routine follow-up. He is doing generally well except for mildly decreased appetite and unintentional loss of 5 lb weight. His CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2 cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirmed the adrenal and liver lesions to be hypermetabolic. In addition, the PET showed multiple FDG avid bone lesions throughout the spine. Brain MRI was negative for any brain metastasis.
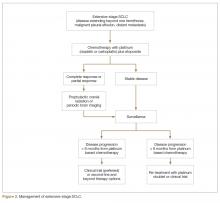
What is the standard of care for extensive-stage SCLC?
For extensive-stage SCLC, chemotherapy is the mainstay of treatment, with the goals of treatment being prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option (Figure 2).
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity [39–42]. Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit [43–45]. The addition of antiangiogenic agent bevacizumab to standard platinum-based doublet has not yielded prolongation of OS in SCLC and led to unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease [46–51]. Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase III trial and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS [52,53].
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
Second-Line Therapy
Despite being exquisitely chemo-sensitive, SCLC is associated with very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse since first-line platinum based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If the disease progression occurs between 3 to 6 months since platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25% [54,55]. A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (cyclophosphamide, adriamycin, vincristine) (Table 2).