Small-Cell Lung Cancer
Case Continued
The patient underwent FDG-PET scan that showed the presence of hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There were no other areas of FDG avidity. His brain MRI did not show any evidence of brain metastasis. Thus, he was confirmed to have limited-stage SCLC.
What is the standard of care for limited-stage SCLC?
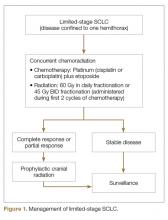
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care for the treatment of limited stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
Choice of Chemotherapy
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen [9]. This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile [10–12]. Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive stage SCLC [13–15]. A meta-analysis of 4 randomized trials, including 663 patients with SCLC, comparing cisplatin-based versus carboplatin-based regimens where 32% of patients had limited stage disease and 68% had extensive stage disease showed no statistically significant difference in the response rate, progression free survival (PFS), or OS between the two regimens [16]. Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the two regimens is different. Cisplatin based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy induced nausea/vomiting [13], while carboplatin-based regimens are more myelosuppressive [17]. In addition, the combination of thoracic radiation with either of these regiments is associated with higher risk of esophagitis, pneumonitis, and myelosuppression [18]. The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation [19]. Of note, intravenous (IV) etoposide is always preferred over oral etoposide, especially in curative setting given unreliable absorption and bioavailability of oral formulations.
Thoracic Radiation
The addition of thoracic radiation to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown 25% to 30% decrease in local failure and 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited stage SCLC [20,21]. Early (with the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS [18,22,23]. The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The ECOG/RTOG randomized trial compared 45 Gy radiation delivered twice daily over a period of 3 weeks with once a day over 5 weeks, concurrently with chemotherapy. The twice a day regimen led to 10% improvement in 5-year OS (26% vs 16%), but higher incidence of grade 3 and 4 adverse events [24]. Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase III trial (CONVERT) compared 45 Gy twice daily with 66 Gy once daily radiation in this setting. This trial did not show any difference in OS. The patients in twice daily arm had higher incidence of grade 4 neutropenia [25]. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often hyperfractionated regimen is not feasible for the patients and many radiation oncology centers. Hopefully the CALBG 30610 study, which is ongoing, will clarify the optimal radiation schedule for limited-stage disease.
Prophylactic Cranial Irradiation
Approximately 75% of patients with limited-stage disease experience disease recurrence and brain is the site of recurrence in approximately half of these patients. Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiation delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases [26–28]. Although individual small studies have not shown survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS [27]. The majority of patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.