Prevention of Type 2 Diabetes: Evidence and Strategies
From the Maimonides Medical Center (Dr. Karam) and the SUNY Downstate Medical Center (Dr. Karam and Dr. McFarlane), Brooklyn, NY.
Abstract
- Objective. To discuss the epidemic of diabetes highlighting the natural history of the disease and the major clinical trials aimed at diabetes prevention in different prediabetic populations around the world.
- Results. Diabetes prevention studies have evaluated various interventions including lifestyle modifications, metformin, alpha-glucosidase inhibitors, thiazolidinediones, nateglinide, and xenical as well as the renin-angiotensin aldosterone system (RAS) inhibitors. Lifestyle modifications seem to be the safest, most effective, and most sustainable intervention to prevent diabetes. Except for metformin, the potential diabetes prevention benefits of the studied pharmacologic agents are limited by safety concerns or lack of durable efficacy or tolerability. RAS blockade and fibrates have a favorable glycemic effect, and, when indicated, are reasonable treatment options for hypertension and hyperlipidemia in prediabetic patients.
- Conclusion. As recommended by American Diabetes Association guidelines, patients with prediabetes should be referred to an intensive diet and physical activity behavioral counseling program; diet and activity goals include a loss of 7% of body weight and at least 150 minutes of moderate physical activity per week. Metformin therapy for diabetes prevention should be considered as well.
Key words: prediabetes; type 2 diabetes mellitus, diabetes prevention, lifestyle modifications.
Diabetes mellitus has reached pandemic proportions across the globe. The International Diabetes Federation (IDF) estimates that in 2015 around 415 million people, or 1 in 11 adults, had diabetes, compared to 285 million in 2010, with 5 million deaths, or 1 death every 6 seconds, occurring because of diabetes or diabetes complications [1]. In the United States, an estimated 29.1 million Americans, or 9.3% of the population, have diabetes, 27.8% of them undiagnosed [2]. The prevalence of diabetes increases significantly with age, affecting around 16.2% of American adults aged 45 to 64 years and 25.9% of adults aged 65 years or older [2]. The Centers for Disease Control and Prevention (CDC) estimates that, with current trends, as many as 1 in 3 American adults could have diabetes by 2050 [3].
Type 2 diabetes mellitus (T2DM) accounts for the majority of prevalent and newly diagnosed diabetes in the world, and is strongly linked to overweight and inactivity in adults [4]. T2DM is increasingly being diagnosed in pediatric patients, in whom type 1 diabetes has historically been predominant; it now accounts for approximately 30% of newly diagnosed diabetes in children aged 10 to 19 years, exceeding 50% in certain ethnicities such as non-Hispanic black and American Indian/Alaska Native children [2].
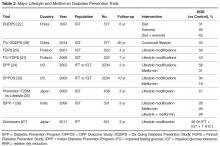
These alarming trends have spurred significant research and public efforts aimed at reducing the prevalence of diabetes by preventing T2DM. Indeed, insulin resistance and abnormal carbohydrate metabolism progress over many years prior to the diagnosis of diabetes and manifest with different clinical and biochemical features. Both the pathophysiology and the natural history of T2DM offer clinicians an opportunity to identify patients at risk for developing the disease and to implement prevention strategies. This article outlines the risk factors and diagnostic criteria for prediabetes, describes the studies that have explored diabetes prevention through lifestyle changes, pharmacotherapy, or surgery, and reviews recommendations for managing patients at risk.
Risk Factors and Screening for T2DM
The American Diabetes Association (ADA) recommends screening all adults for prediabetes by assessing for diabetes risk factors [8]. Glucose testing is recommended in individuals aged 45 years or older, and should be considered in adults of any age who are overweight or obese (body mass index [BMI] ≥ 25 kg/m2 or ≥ 23 kg/m2 in Asian Americans) and have 1 or more additional risk factors for diabetes. Testing also should be considered in children and adolescents who are overweight or obese and who have 2 or more additional risk factors. If tests are normal, repeat testing carried out at a minimum of 3-year intervals is suggested [8].
Prediabetes
Abnormalities in glucose metabolism progress along a continuum through various stages before T2DM develops. Years before the development of overt diabetes, and especially in the presence of excessive visceral fat, cellular sensitivity to insulin gradually decreases, leading to a compensatory increased insulin secretion [9]. With time, and under continuous increased demand, pancreatic beta cell function declines and ultimately fails to overcome insulin resistance and maintain a normal glucose metabolism, resulting in prediabetes followed by the development of diabetes. This early beta cell dysfunction was illustrated by the decreased beta cell volume observed on autopsy of obese patients with IFG or T2DM, when compared to obese individuals with normal glucose tolerance [10]. It is estimated that around 40% to 70% of beta cell function is already lost by the time diabetes is clinically diagnosed. This relatively slow pathophysiologic process allows the identification of at-risk patients well before their blood glucose levels reach the diabetic diagnostic thresholds, and therefore presents an opportunity for prevention.
Diagnostic Criteria
The ADA guidelines released in 2003 define prediabetes as IFG (fasting blood glucose [FBG] levels of 100–125 mg/dL), IGT (glucose levels of 140–199 mg/dL at 2 hours during an oral glucose tolerance test [OGTT] following an oral load of 75 g of dextrose), or both. Additionally, hemoglobin A1C (A1C) was introduced as a diagnostic tool for prediabetes in 2010, with values between 5.7% and 6.4% indicating prediabetes [8]. Most of these thresholds were chosen due to their association with increased rates of complications, notably retinopathy and cardiovascular disease.
A combined report from the World Health Organization (WHO) and the IDF published in 2006 defined intermediate hyperglycemia as IFG, but with a higher cutoff for FBG (110–125 mg/dL) than the ADA’s definition, and/or IGT (2-hour OGTT glucose level of 140–199 mg/dL) [11]. The rationale for a higher cut-point for IFG is the concern about the increased prevalence of IFG and its impact on individuals and health systems and the more favorable cardiovascular risk profile and decreased risk of progression to diabetes in the group of patients with FBG of 100 to 110 mg/dL when compared to the group with FBG of 110 to 125 mg/dL. The report does not recommend the use of A1C in the diagnosis of diabetes or intermediate hyperglycemia because of a lack of global consistency and the potential for other factors that can be prevalent in some developing countries, such as hemoglobinopathies and anemia, to interfere with the assay.
Prevalence and Progression to Diabetes
According to CDC data from 2014, up to 86 million American adults, more than 1 in 3, have prediabetes, and 9 out of 10 of these individuals are undiagnosed [2]. It is estimated that approximately 25% of people diagnosed with either IFG or IGT progress to diabetes mellitus over a 3- to 5-year period [12]. If observed for longer periods, most prediabetic persons will probably develop diabetes. The highest rate of progression to diabetes is observed in patients with both IFG and IGT, older age, overweight, or other diabetic risk factors.
Complications
In addition to increasing the risk for progression to diabetes, prediabetes is independently associated with microvascular and macrovascular complications and increased risk of death, prior to the actual onset of diabetes. The DECODE study demonstrated significantly increased mortality in 2766 individuals with IGT after 7 years of follow-up, when compared to normoglycemic patients; this effect was more prominent in participants with IGT than in participants with IFG [13]. In the Australian Diabetes, Obesity and Lifestyle Study, IFG was found to be an independent predictor for cardiovascular mortality after adjustment for age, sex, and other traditional cardiovascular risk factors [14].
Similarly, a recent meta-analysis demonstrated that the presence of IFG was significantly associated with future risk for coronary heart disease (CHD), with the risk increase starting when fasting plasma glucose was as low as 100 mg/dL; however, this finding may have been confounded by the presence of undetected IGT or other cardiovascular risk factors [15]. Another recent systematic review of 53 prospective cohort studies with 1,611,339 participants showed that prediabetes (IFG or IGT) was associated with an increased risk of composite cardiovascular disease, CHD, stroke, and all-cause mortality [16].
The association between retinopathy and prediabetes has been described in multiple reports and this association has helped guide authors on selected thresholds for diagnosis of prediabetes. For example, in 1 study, the incidence of retinopathy in individuals with IGT was 12% among Pima Indians [17]. Similarly, in a follow-up study of the Diabetes Prevention Program, 8% of prediabetic participants who remained nondiabetics had evidence of retinopathy [18].
Neuropathy also has been observed in prediabetes. A noninvasive neurologic evaluation of individuals with IGT revealed subclinical neural dysfunction suggestive of cardiovascular autonomic neuropathy [19]. At the clinical level, a study that evaluated 100 patients with chronic idiopathic axonal neuropathy of unknown etiology found IFG in 36 and IGT in 38 patients, underscoring the role of abnormal glucose metabolism in these patients [20].
Nephropathy may also be more prevalent in those with prediabetes. In a 1999–2006 National Health and Nutrition Examination Survey analysis, the adjusted prevalence of chronic kidney disease, defined by estimated glomerular filtration rate (eGFR) of 15 to 59 mL/min per 1.73 m2 or albumin-creatinine ratio ≥ 30 mg/g, was 17.1% in individuals with IFG, compared to 11.8% in individuals with normal fasting glucose [21].