Adverse events from systemic treatment of cancer and patient-reported quality of life
Background Treatment-related adverse events (AEs) have a negative impact on the quality of life (QoL) of cancer patients. Patient and general public views can help in the investigation of patient needs and preferences.
Objective To compare the impact on QoL reported by cancer patients who have experienced a particular AE with that envisioned by general public participants (hypothetical patients) who have not experienced AEs.
Methods Five AEs were selected: total alopecia (Common Terminology Criteria for Adverse Events [CTCAE] grade 2), acneiform rash (CTCAE, grade 1 and grades 2-4), oxaliplatin-associated peripheral neuropathy (oxaliplatin-specific scale, grades 1, 2, and 4); diarrhea and vomiting (CTCAE, grades 1-2 and grades 3-4), resulting in 10 toxicity variables. Cancer patients and general public participants completed the Visual Analog Scale (VAS; 0 = poorest QoL; 100 = better QoL), and cancer patients also completed the EQ-5D-5L questionnaire (full range for Spanish population, -0.654-1.000).
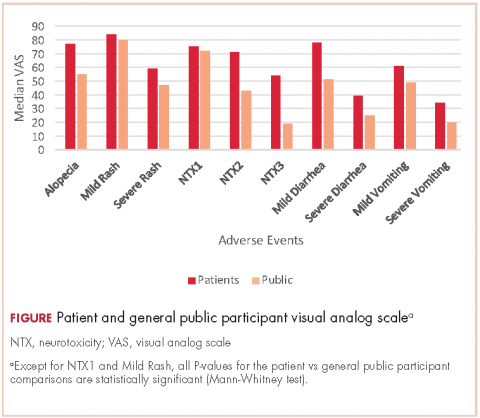
Results 246 general public participants and 200 toxic events in 139 cancer patients were analyzed. For all 10 endpoints, the mean VAS was higher for patients than for the general public participants. That difference was statistically significant (Mann-Withney U test) for all endpoints except for grade 1 neuropathy and grade 1 rash. For both groups, alopecia had a lower impact on quality of life than did severe rash (mean VAS for patients, 77 [alopecia] vs 59 [rash], compared with 55 vs 47, respectively, for the general public group). There was a positive linear correlation between the EQ-5D-5L and VAS (Spearman rho, 0.681; P = .001).
Limitations Patients were asked to score AEs as separate and encapsulated from other concurrent symptoms. This exercise may be rather difficult for patients.
Conclusions The impact of therapy-related AEs on QoL was lower in patients who have experienced the AEs than it was for the general public participants who had not experienced the AEs. The EQ-5D-5L is a useful tool for evaluating AEs.
Funding/sponsorship Provided by the Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain.
Accepted for publication July 14, 2017
Correspondence Vicente Valentí, PhD; vvalenti@xarxatecla.cat
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(5):e256-e262
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0364
Submit a paper here
Survey procedures
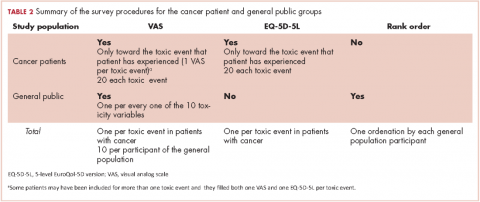
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
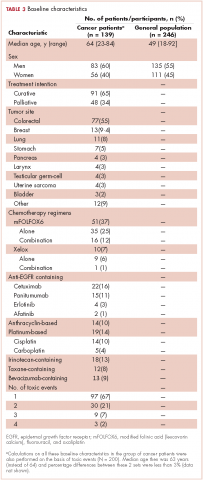
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
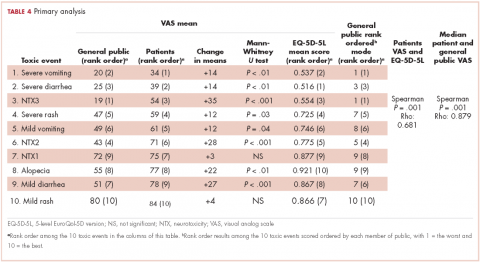
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.