Reducing the risk of breast cancer: A personalized approach
Although primary care physicians often use the same screening schedule and preventive measures for all the women they see, optimal breast cancer risk reduction requires an individualized approach.
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
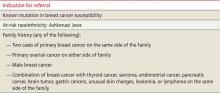
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at https://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to https://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from https://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).