Battling influenza: Changes for the 2012-2013 season
A revised vaccine algorithm aids in decision making for children ages 6 months to 8 years; a newly approved quadrivalent LAIV should be available for the 2013-2014 season.
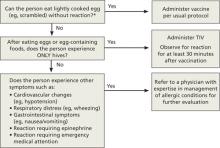
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (https://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |