It’s time to rethink your approach to C diff infection
Metronidazole is no longer the drug of choice for first-line therapy. And fecal microbiota transplantation has proven effective for certain patients.
PRACTICE RECOMMENDATIONS
› Keep in mind that previous exposure to antibiotics is the most important risk factor for initial and recurrent Clostridioides difficile infection (CDI). Thus, appropriate antimicrobial stewardship is key to prevention. C
› Begin with vancomycin or fidaxomicin (over metronidazole) for first-line treatment of CDI in adults. A
› Consider fecal microbiota transplantation in high-risk patients with recurrent CDI for whom antimicrobial therapy has failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
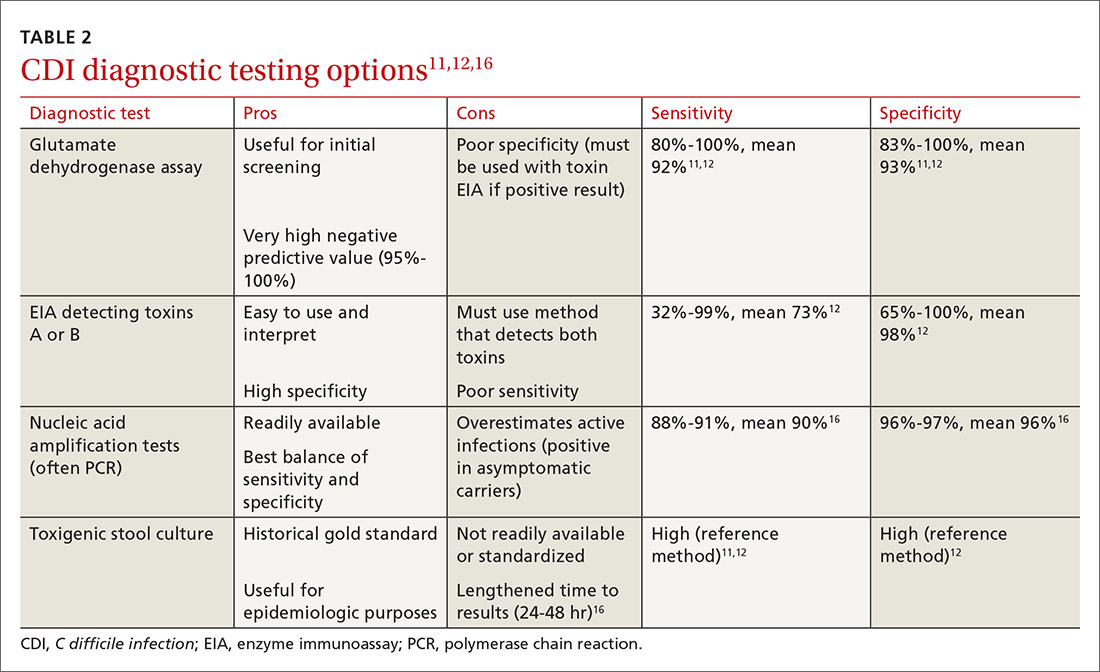
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed






