Influenza update
Consider starting vaccination in September or later to avoid waning immunity by the end of the flu season.
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
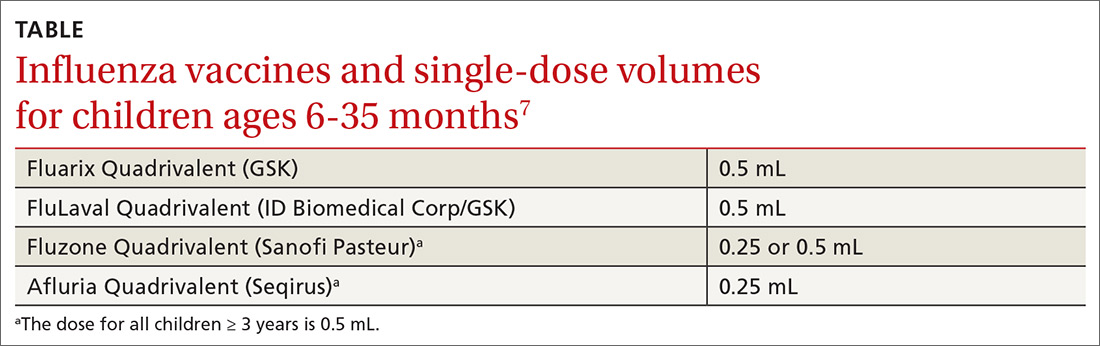
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7
All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8






