Reflectance Confocal Microscopy: An Effective Diagnostic Tool for Dermatophytic Infections
Current methods for diagnosing dermatophytic infections have various drawbacks. Analysis via skin scrapings and biopsies can be invasive and/or take too long to yield results. Reflectance confocal microscopy (RCM) is an emerging in vivo imaging technology that can potentially be used to diagnose cutaneous dermatophytic infections. This modality provides high-resolution images of the skin extending to the level of the superficial reticular dermis that could reveal the presence of fungal hyphae. In this retrospective chart review, we investigated the application of RCM as a diagnostic tool in the setting of a private practice. Images were used to diagnose dermatophyte infections and the results were compared to those of other established diagnostic methods. We found RCM to be a potentially effective and highly sensitive tool in the diagnosis of cutaneous dermatophytic infections.
Practice Points
- Current methods for diagnosing dermatophytosis can be invasive, with variable sensitivity and/or slow turnaround time.
- Reflectance confocal microscopy is a promising option for rapid noninvasive diagnosis of dermatophytosis.
Comment
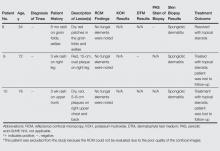
In this chart review, we evaluated the utility of using RCM in the diagnosis of dermatophytic infections of the skin by comparing findings noted on confocal imaging with those of other methods of diagnosis (Table). We included cases in which the clinical presentation raised the possibility of dermatophytic infection. Cases were considered positive for dermatophytes if KOH preparation, fungal culture, or skin biopsy (with or without periodic acid–Schiff staining) were positive or if there was a complete response to antifungal treatment alone. In this small number of cases, we found that RCM was 100% sensitive, as hyphae were readily seen in all cases of dermatophytic infections. In 1 RCM-positive case (patient 3), fungal culture with DTM was negative, but antifungal therapy was nonetheless given. Because the lesion resolved promptly with econazole, RCM proved to be true positive and DTM proved to be false negative (Table). Reflectance confocal microscopy imaging, however, was less specific. Of the 5 cases that showed no presence of dermatophytic infection, there was 1 case (patient 6) in which the pathologist could recognize structures that resembled fungal hyphae. There are various possible sources of structures masquerading as dermatophytes on confocal imaging, including the edges of nonnucleated loose keratinocytes, keratin fragments, and other foreign fibers. Evaluation by an experienced investigator can certainly help in limiting false-positive analyses, but a larger case study would be useful to develop a set of specific criteria to aid in the differentiation of fungal hyphae from other artifacts as well as to further define the sensitivity and specificity of RCM.
We also encountered difficulties with the technical aspects of RCM. One case (patient 5) was excluded from the analysis because the images were poor quality and could not be interpreted, and 2 cases (patients 2 and 7) had suboptimal images, in part due to operator error and in part due to equipment error that was recognized later on. The technical difficulties were problematic because no immediate review of image quality was available while patients were still present for possible reimaging. All of the images evaluated in this study were captured shortly after the RCM device was introduced to the practice. It is possible that with more training and a quick, on-site review of image quality, these technical problems could be avoided. Imaging protocols (ie, numbers and levels of scans taken by the confocal microscope) also could be adjusted so they include a large enough range to compensate for potential operator errors; however, these adjustments also could increase overall imaging time.