Calcipotriene 0.005%–Betamethasone Dipropionate 0.064% Ointment Versus Topical Suspension in the Treatment of Plaque Psoriasis: A Randomized Pilot Study of Patient Preference
Vehicle formulation plays a major role in patient adherence to topical psoriasis treatments. The objective of this study was to conduct a preliminary assessment of patient preference for ointment versus topical suspension formulations of calcipotriene 0.005%–betamethasone dipropionate 0.064% for treatment of plaque psoriasis. In our small cohort of 20 participants with mild to moderate plaque psoriasis, the topical suspension formulation was preferred over the ointment, though the difference was not statistically significant (P=.32). Overall, the topical suspension was rated as moderately appealing, while the ointment was rated as slightly appealing (P=.06). Subgroup analyses were limited due to the small sample size. The results of this study may provide clinicians with an alternative topical treatment of plaque psoriasis that provides the benefits of a combination product. In clinical practice, it may be best to offer patients both formulations and they can choose the product that is right for them.
Practice Points
- Patient preference plays an important role in adherence to treatment regimens in chronic skin diseases such as psoriasis.
- The topical suspension formulation of calcipotriene 0.005%–betamethasone dipropionate 0.064% was preferred by psoriasis patients over the ointment; however, the availability of the 2 formulations provides patients with options and allows them to choose which product they prefer.
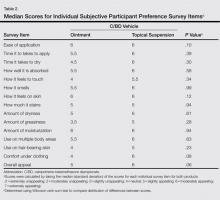
The mean (SD) total preference score for the calcipotriene 0.005%–betamethasone dipropionate 0.064% ointment formulation was 73.5 (19.4) and 80 (20.2) for the topical suspension formulation. The difference between means was -6.5 (95% confidence interval, -19.7-6.8; P=.32 after adjusting for possible carryover and period effects). Participants used a mean (SD) of 11 (11.4) g of product per study arm for the ointment formulation and 8.8 (6.6) g for the topical suspension formulation. There was no correlation between the amount of product used and preference for product (Spearman r=-0.01; P=.94). No statistically significant difference in product preference among men versus women was noted when considering total preference score or median scores of individual survey questions. Median overall appeal rating for the ointment formulation was 5 (slightly appealing) versus 6 (moderately appealing) for the topical suspension formulation, approaching statistical significance with P=.06 (Table 2). No significant carryover effects from one product to the other were noted (P=.64). The mean (SD) total preference scores were 81.1 (18.4) and 77.6 (21.2) in participants who used the topical suspension first followed by the ointment. In participants who used the ointment first followed by the topical suspension, the mean (SD) total preference scores were 69.4 (17.6) and 78.9 (22.8). Self-reported treatment adherence according to the participant’s daily medication diary was 100%.
Adverse effects during the study included 1 report of neck and back muscle pain and 1 report of sinusitis; neither was considered to be related to the study drugs.