Treat-to-Target Outcomes With Tapinarof Cream 1% in Phase 3 Trials for Plaque Psoriasis
The National Psoriasis Foundation (NPF) treatment targets aim to achieve 1% or lower body surface area (BSA) affected after 3 months of treatment. European psoriasis treatment guidelines aim to achieve similar goals based on improvements in Psoriasis Area and Severity Index (PASI) scores. We performed pooled analyses of the PSOARING phase 3 program, which evaluated treat-to-target outcomes for patients treated with tapinarof cream 1% once daily (QD) for up to 52 weeks. Our analyses included 915 patients from PSOARING 1 and PSOARING 2 who had Physician Global Assessment (PGA) scores of 2 or higher before undergoing treatment with tapinarof, including those who received the vehicle in PSOARING 1 and PSOARING 2 and then tapinarof in PSOARING 3. The treatment targets we analyzed included the proportion of patients achieving an absolute BSA of 1% or lower or an absolute total PASI score of 3 or lower. In total, 40% of patients achieved the stringent NPF target of BSA of 1% or lower within 3 months, and 61% achieved a BSA of 1% or lower at any time (median, ~4 months). Furthermore, 75%, 67%, and 50% achieved PASI scores of 3, 2, and 1 or lower, respectively, at any time (median, ~2–6 months). Our results indicated that a high percentage of patients with mild to severe psoriasis can achieve and exceed ambitious treatment targets when treated with topical tapinarof monotherapy for up to 1 year.
Practice Points
- In clinical practice, many patients with psoriasis do not achieve treatment targets set forth by the National Psoriasis Foundation and the European Academy of Dermatology and Venereology, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.
- Tapinarof cream 1% is a nonsteroidal aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults; it also is being studied for the treatment of plaque psoriasis in children 2 years and older.
- Tapinarof cream 1% is an effective topical treatment option for patients with plaque psoriasis of any severity, with no limitations on treatment duration, total extent of use, or application sites, including intertriginous skin and sensitive areas.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18
COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
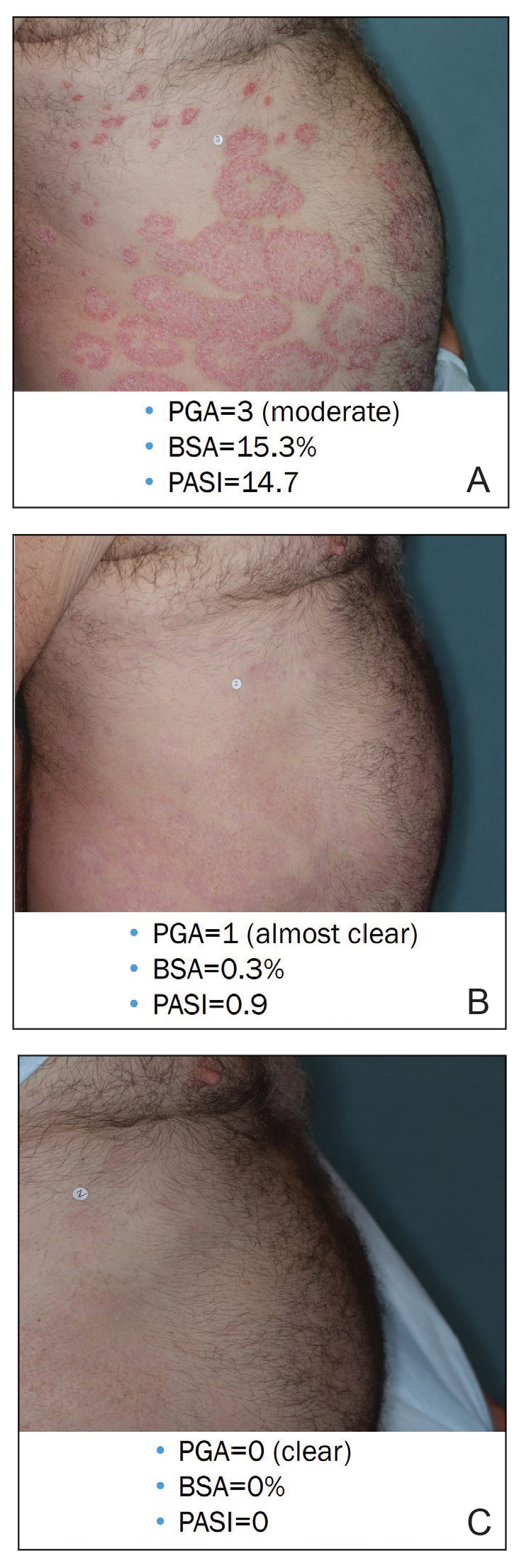
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.






