Long-term Remission of Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is an autoinflammatory condition that is poorly characterized in the literature, and achieving extended remission has proven difficult. We report the case of a patient with a history of cystic acne and HS who was referred to dermatology for evaluation of refractory PG of 18 months’ duration. After therapeutic refinement, remission was achieved and maintained for 4 years using adalimumab and cyclosporine. Treatment success generally is only achieved using multimodal therapies targeting various molecules of the innate immune system, suggesting that PASH syndrome may involve multiple pathways of the inflammatory cascade; however, current understanding of the disease mechanism is scarce. The distinct genotypic and immunologic abnormalities of PASH syndrome require further study to uncover the mechanisms that therapeutic regimens aim to interrupt. Multimodal therapy is necessary to control the autoinflammatory nature of this disease, and further reports of therapeutic successes are essential for advancement in understanding this debilitating condition.
Practice Points
- Despite phenotypic similarities among pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (PASH) syndrome; pyogenic arthritis, PG, and acne syndrome; and pyogenic arthritis–PASH syndrome, there are genotypic differences that contribute to unique inflammatory cytokine patterns and the need for distinct pharmacologic considerations within each entity.
- When formulating therapeutic regimens for patients with PASH syndrome, it is essential for dermatologists to consider the likelihood of hyperactivity in multiple pathways of the innate immune system and utilize a combination of multimodal antiinflammatory therapies.
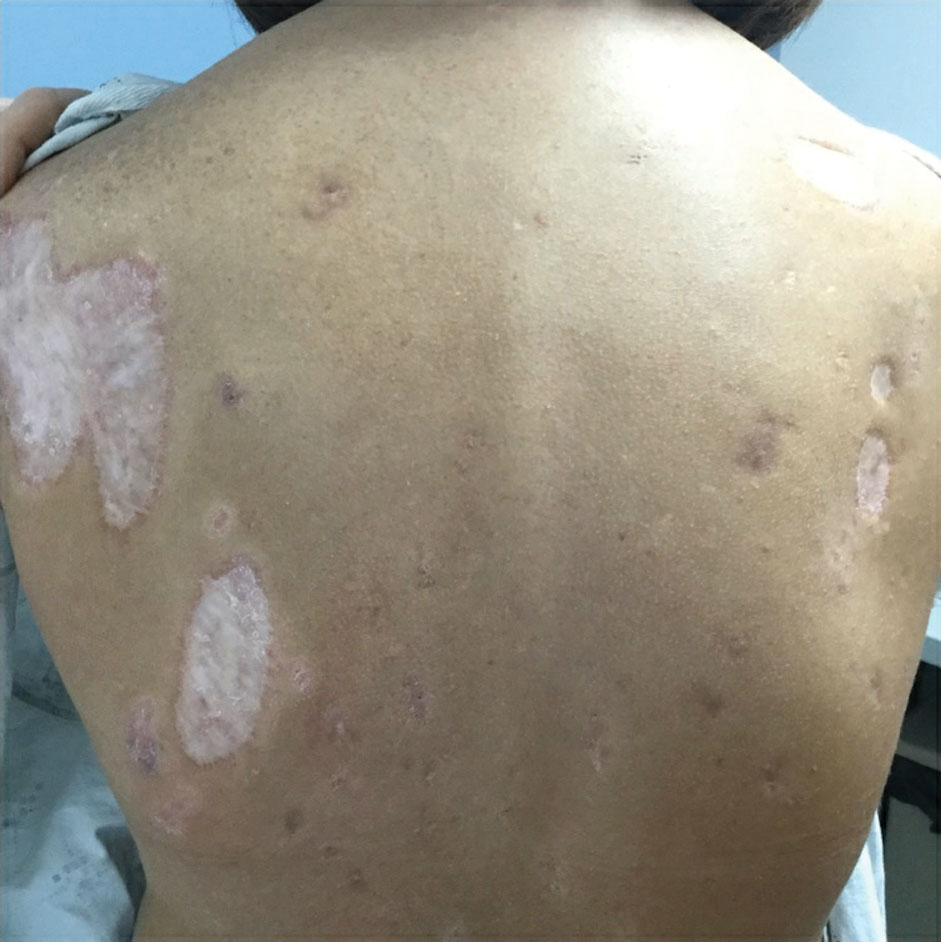
The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.
Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3






