Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a chronic form of skin cancer. Skin-directed therapies rarely achieve complete clearance of lesions, and recurrences are frequent. In this case series, 9 patients with stage IA to IVA2 CTCL received intralesional (IL) therapy with 5-fluorouracil (5-FU) and imiquimod (IMQ) cream 5% daily to recalcitrant plaques and tumors. All 9 patients attained a complete response (CR) with no recurrences reported and no severe side effects. We find that combination IL 5-FU and IMQ cream 5% daily is a well-tolerated, effective, and durable skin-directed therapy for recalcitrant plaques and tumors in CTCL.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea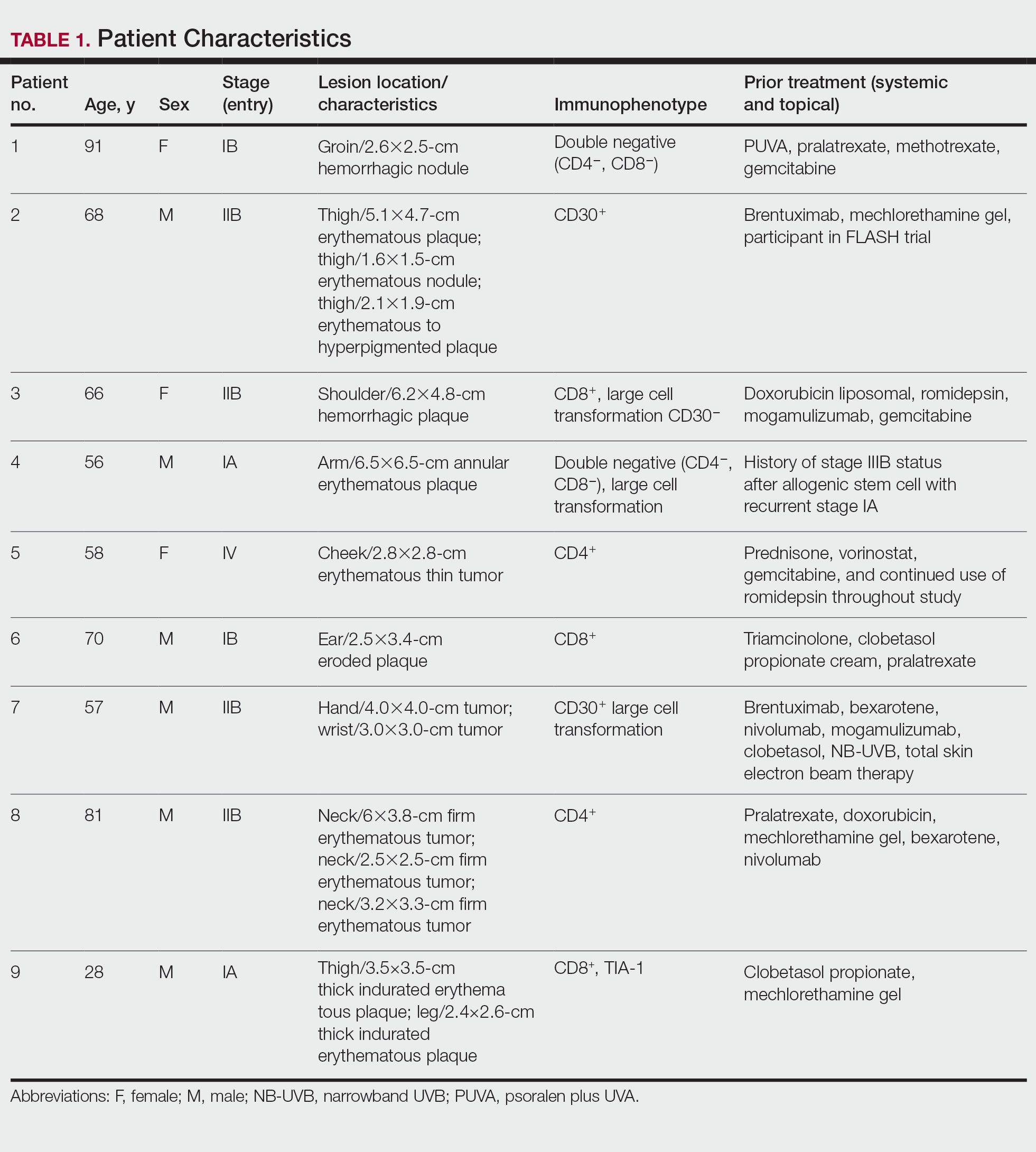
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
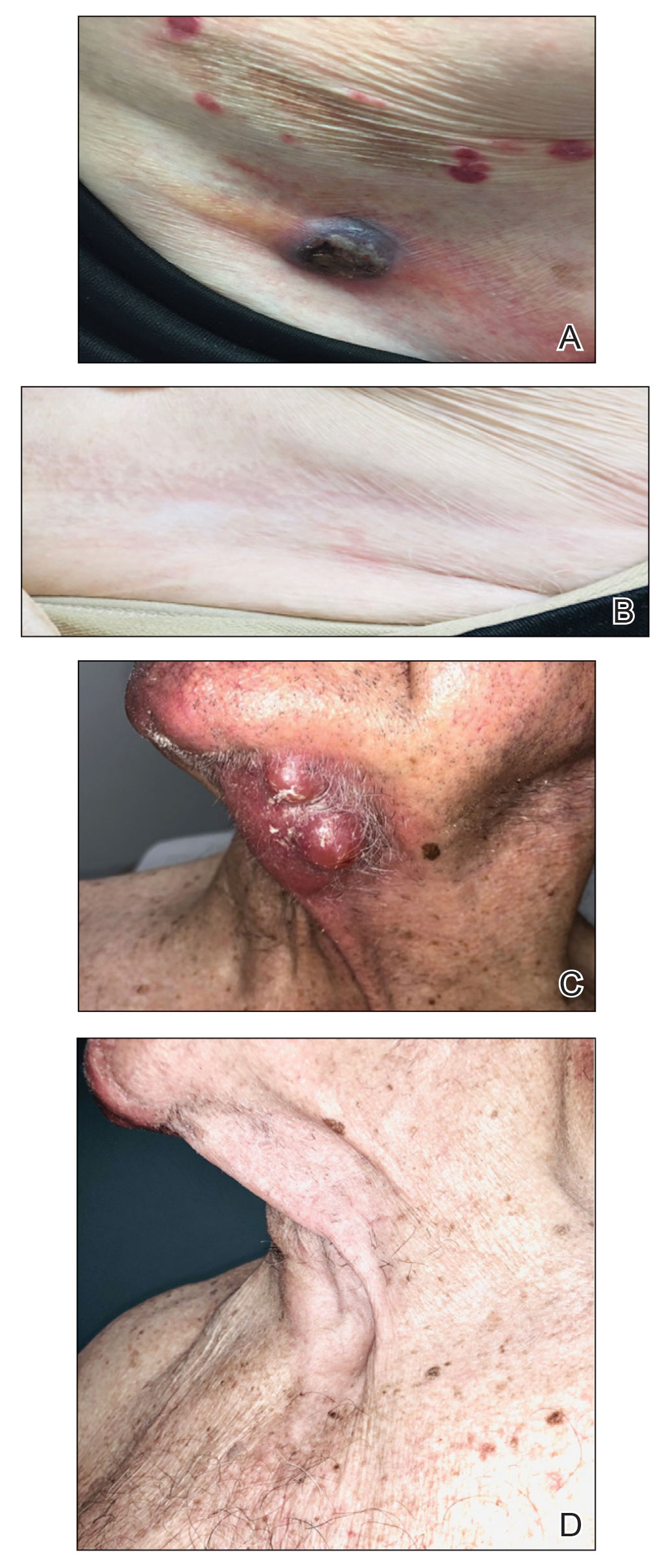
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.






