New Razor Technology Improves Appearance and Quality of Life in Men With Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB) consists of ingrown hairs leading to papules, pustules, and discomfort. This prospective, 12-week clinical study aimed to assess the appearance of males with razor bumps and shaving irritation when using a new razor technology with 2 blades separated by a bridge feature (SkinGuard [Procter & Gamble]). The impact on participants’ shave-related itching, burning, and stinging severity, as well as quality of life (QOL), also was assessed. In men with PFB, shaving with the test razor at least 5 times per week over a 12-week period improved the appearance and QOL of males with razor bumps and shaving irritation compared with baseline.
Practice Points
- Pseudofolliculitis barbae (PFB) is a common follicular inflammatory disorder associated with shaving, most commonly seen in men of African ancestry. It can be distressing and cause a substantial impact on quality of life (QOL).
- Frequent use of a novel razor technology designed specifically for men with PFB was found to improve skin appearance and QOL after 12 weeks vs baseline.
- This razor technology provides an alternative approach to help manage PFB for men who wish to or need to continue shaving.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
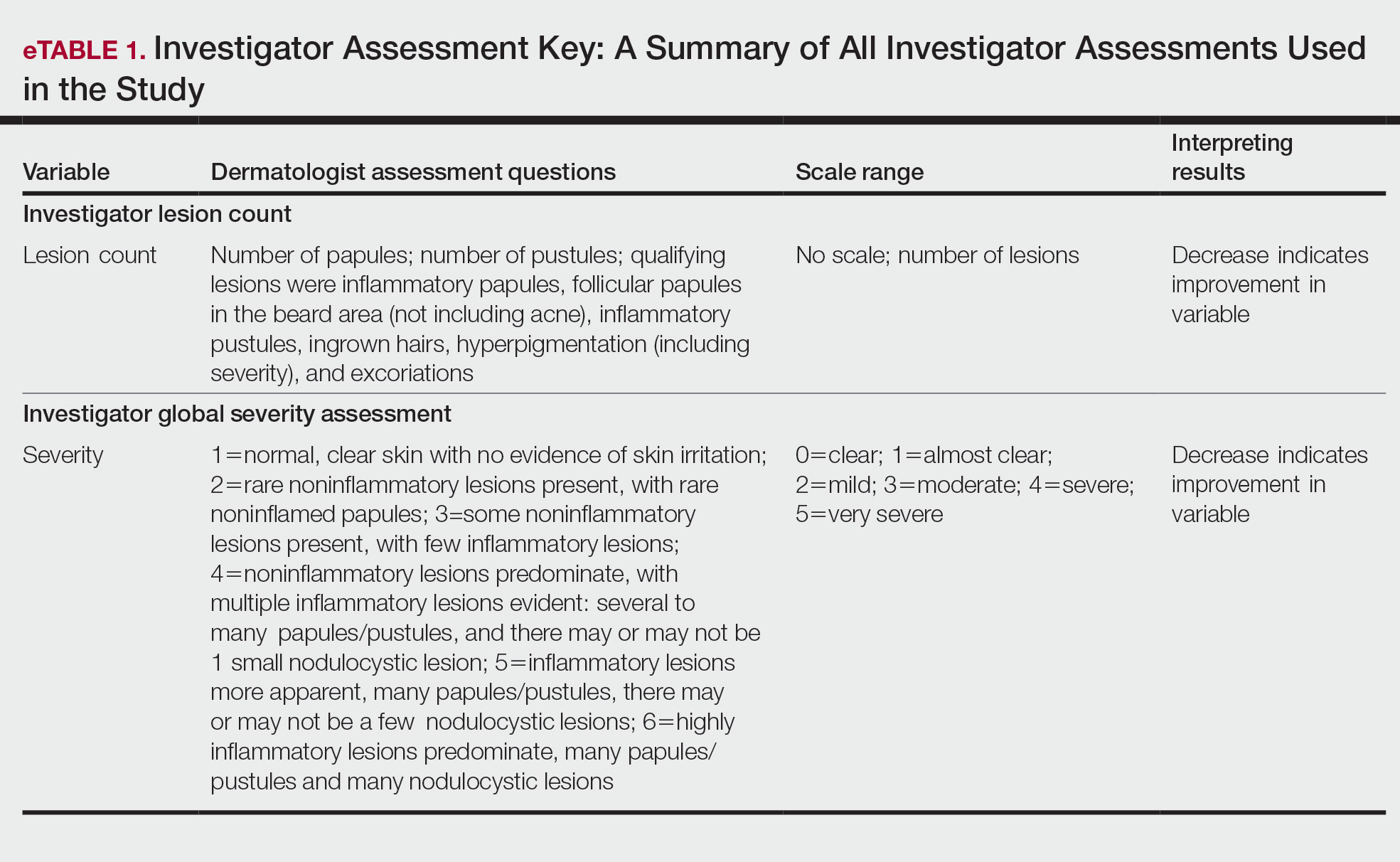
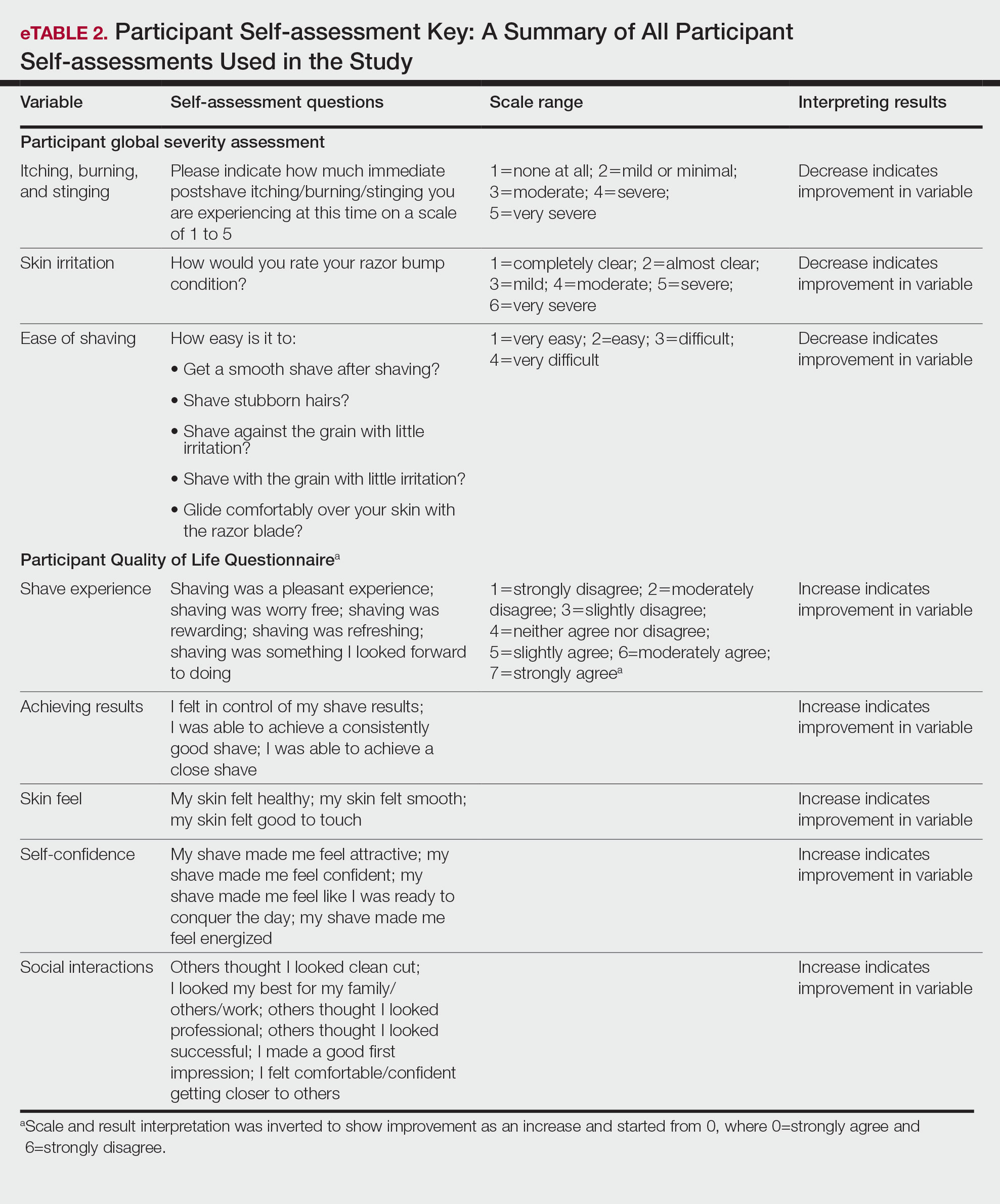
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.
The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.
Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.






