Allergic Reaction to Vanadium Causes a Diffuse Eczematous Eruption and Titanium Alloy Orthopedic Implant Failure
Allergy as a cause of adverse outcomes in patients with implanted orthopedic hardware is controversial. Allergy to titanium-based implants has not been well researched, as titanium is traditionally thought to be inert. We highlight the case of a patient who developed systemic dermatitis and implant failure after surgical placement of a titanium alloy (Ti6Al4V) plate in the left foot. The hardware was removed and the eruption cleared in the following weeks. The plate and screws were submitted for metal analysis. The elemental composition of both the plate and screws included 3 major elements—titanium, aluminum, and vanadium—as well as trace elements. Metal analysis revealed that the plate and screws had different microstructures, and electrochemical studies demonstrated that galvanic corrosion could have occurred between the plate and screws due to their different microstructures, contributing to the release of vanadium in vivo. The patient was patch tested with several metals including components of the implant and had a positive patch test reaction only to vanadium trichloride. These findings support a diagnosis of vanadium allergy and suggests that clinicians should consider including vanadium when patch testing patients with a suspected allergic reaction to vanadium-containing implants.
Practice Points
- Vanadium may be an underrecognized allergen in patients with metal implants.
- Consider vanadium allergy in those with surgical implants and signs of hypersensitivity reaction.
- Test for allergy with vanadium trichloride.
- Niobium is an alternative for implants in vanadium-allergic patients.
After the hardware was removed, it was analyzed to determine the elemental composition of the plate and screws, and the patient was then patch tested with the major metal components of the implant: aluminum chloride hexahydrate 2.0% pet, elemental titanium 10.0% pet, titanium dioxide 10.0% pet, titanium (III) nitride 5.0% pet, titanium (III) oxalate decahydrate 5.0% pet, elemental vanadium 5.0% pet, and vanadium (III) chloride 1.0% pet. She demonstrated a 1+ reaction (erythema and induration) to vanadium trichloride at 72 and 96 hours.
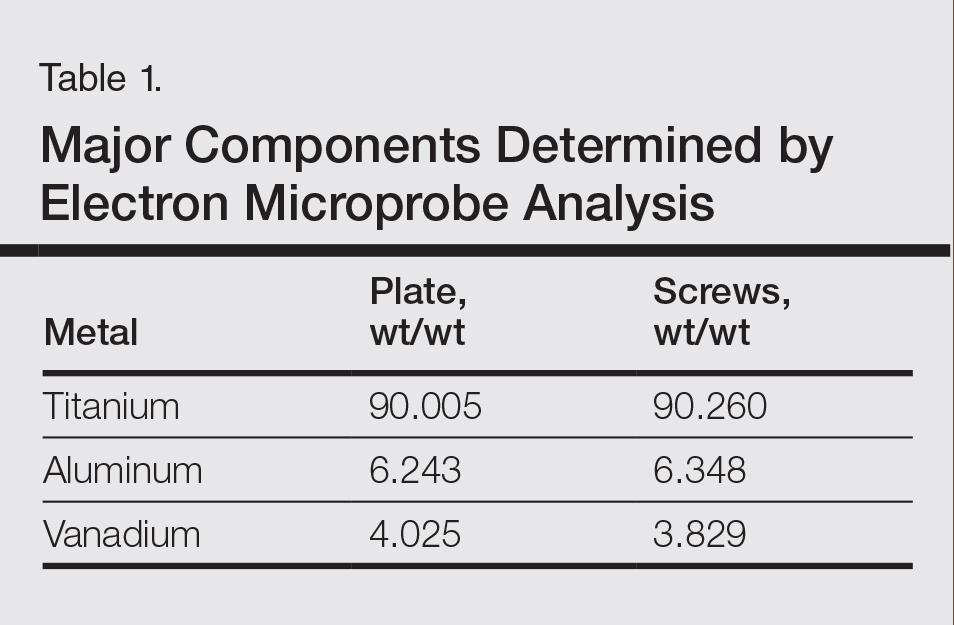
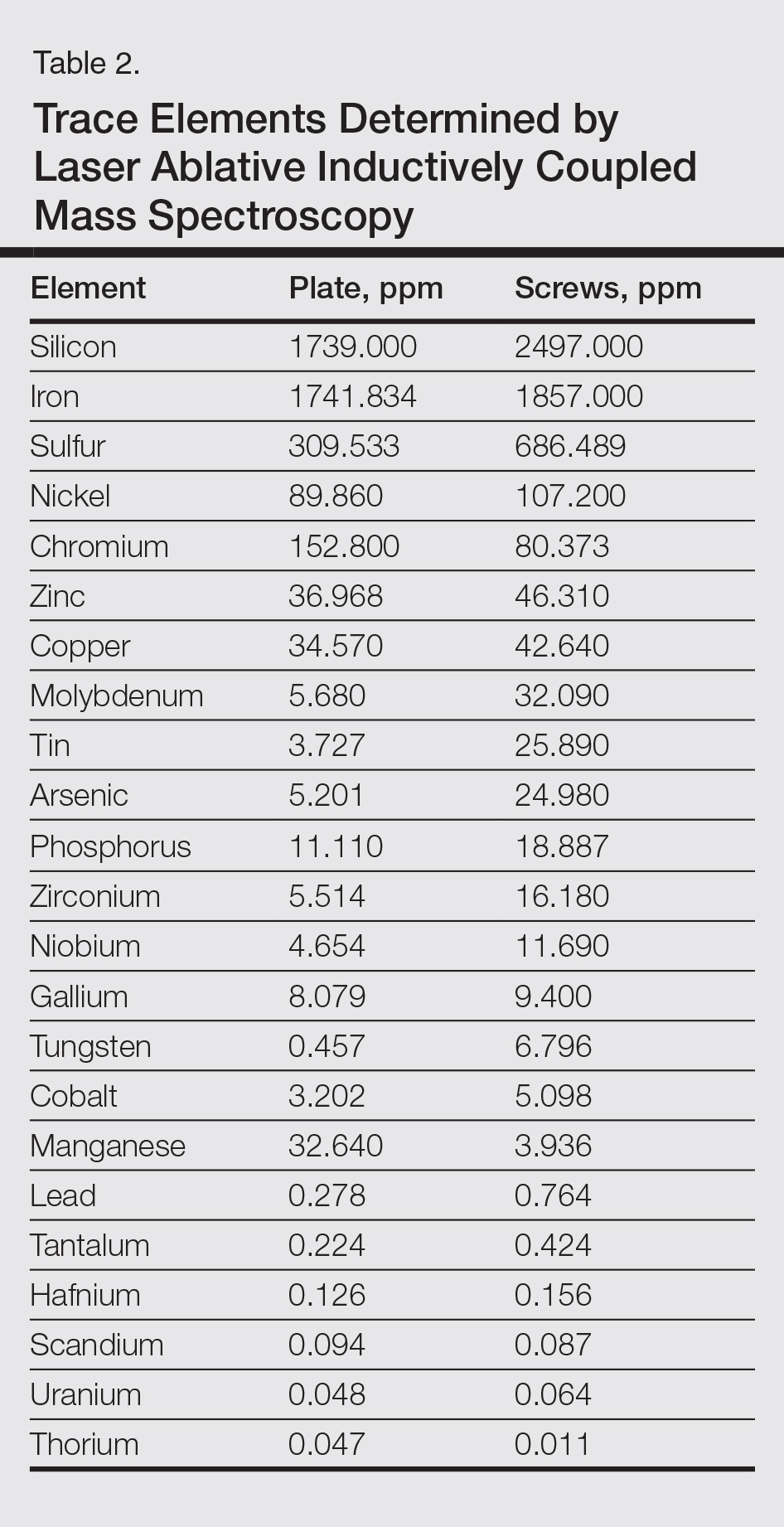
The plate and screws removed from the patient were sterilized and submitted for analysis. Electron microprobe analysis confirmed that the major elemental composition of the plate and screws essentially matched the manufacturer’s listing (Table 1). The trace elements were determined using laser ablative inductively coupled mass spectroscopy, which demonstrated that the screws were of different metal composition from the plate (Table 2). Electron microprobe analysis also was used to determine the microstructure of the plate and screws. The plate had 2 distinct phases consisting of a titanium-aluminum phase and a vanadium phase, whereas the screw was much more homogeneous. Basic electrochemical studies were performed in a salt solution replicating the tissue of the foot. These studies showed that galvanic corrosion could have occurred between the plate and screws due to the differences of composition.