Over-the-counter and Natural Remedies for Onychomycosis: Do They Really Work?
Onychomycosis is a fungal infection of the nail unit that may lead to dystrophy and disfigurement over time. It accounts for up to 50% of all nail conditions, with toenails affected more commonly than fingernails. Onychomycosis may affect quality of life and increase the prevalence and severity of foot ulcers in patients with diabetes. Available oral agents approved by the US Food and Drug Administration (FDA) for the treatment of onychomycosis include terbinafine and itraconazole, which have demonstrated good efficacy but are associated with the risk of systemic side effects and drug-drug interactions. Topical medications that are FDA approved for onychomycosis include ciclopirox, efinaconazole, and tavaborole. These therapies generally have incomplete efficacy compared to systemic agents as well as long treatment courses and possible local side effects such as erythema and/or blisters. Given the need for safe, effective, and cost-effective options for onychomycosis therapy, there has been a renewed interest in natural and over-the-counter (OTC) alternatives. This review will synthesize the laboratory data, known antifungal mechanisms, and clinical studies assessing the efficacy of OTC and natural products for onychomycosis treatment.
Practice Points
- Natural remedies, including tea tree oil, natural topical cough suppressants, natural coniferous resin lacquer, Ageratina pichinchensis extract, and ozonized sunflower oil, have shown antifungal activities in in vitro studies.
- Some of these products have efficacy and appear to be safe in clinical studies.
- Larger randomized clinical trials demonstrating efficacy are required before we can recommend these products to our patients.
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
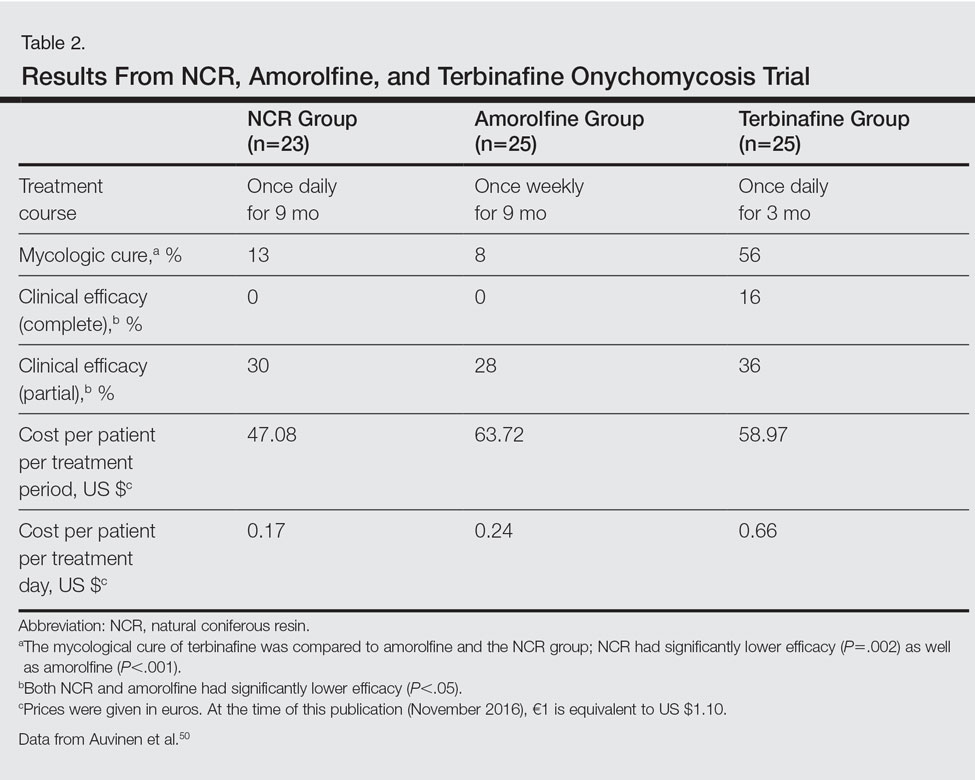
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50