When Do Efficacy Outcomes in Clinical Trials Correlate With Clinical Relevance? Analysis of Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel in Moderate to Severe Acne Vulgaris
Acne vulgaris (AV) is a common skin disease that is challenging to successfully treat due to its complex underlying pathophysiology and chronicity. Unrealistic expectations based on the desire for rapid and complete clearance or local tolerability reactions related to topical medications often lead to incomplete adherence with therapy, premature treatment cessation, and poor therapeutic outcomes. Despite stressing to patients the importance of compliance and the lag time of several weeks before visible improvement may be noted with treatments for AV, data on evaluation of the time taken to achieve a clinically meaningful improvement of AV that may be perceived by clinicians and patients are limited. Clindamycin phosphate 1.2%–benzoyl peroxide 3.75% (clindamycin-BP 3.75%) gel has been shown in pivotal trials to be effective and well tolerated in patients with moderate to severe AV. This article reviews a new concept referred to as time to onset of action (TOA), which is described in detail and illustrated using the pivotal trial data with clindamycin-BP 3.75% gel for treatment of AV.
Practice Points
- Time to onset of action (TOA) refers to how long it takes after starting a therapy for a patient to perceive visible improvement.
- Time to onset of action has been determined based on data to date to correlate overall with a 25% lesion reduction.
- The TOA for clindamycin phosphate 1.2%–benzoyl peroxide 3.75% gel applied once daily based on analysis of pivotal trial data is 3 weeks or less depending on the severity of acne vulgaris at baseline.
Efficacy evaluations included inflammatory and noninflammatory lesion counts and EGSS at screening, baseline, and during treatment (weeks 4, 8, and 12).10 Primary efficacy end points included absolute change in mean inflammatory and noninflammatory lesion counts and the proportion of patients who achieved at least a 2-grade reduction in EGSS from baseline to week 12 (treatment success at end of study). Secondary efficacy end points included mean percentage change from baseline to week 12 in inflammatory and noninflammatory lesion counts and the proportion of patients who considered themselves clear or almost clear at week 12.10
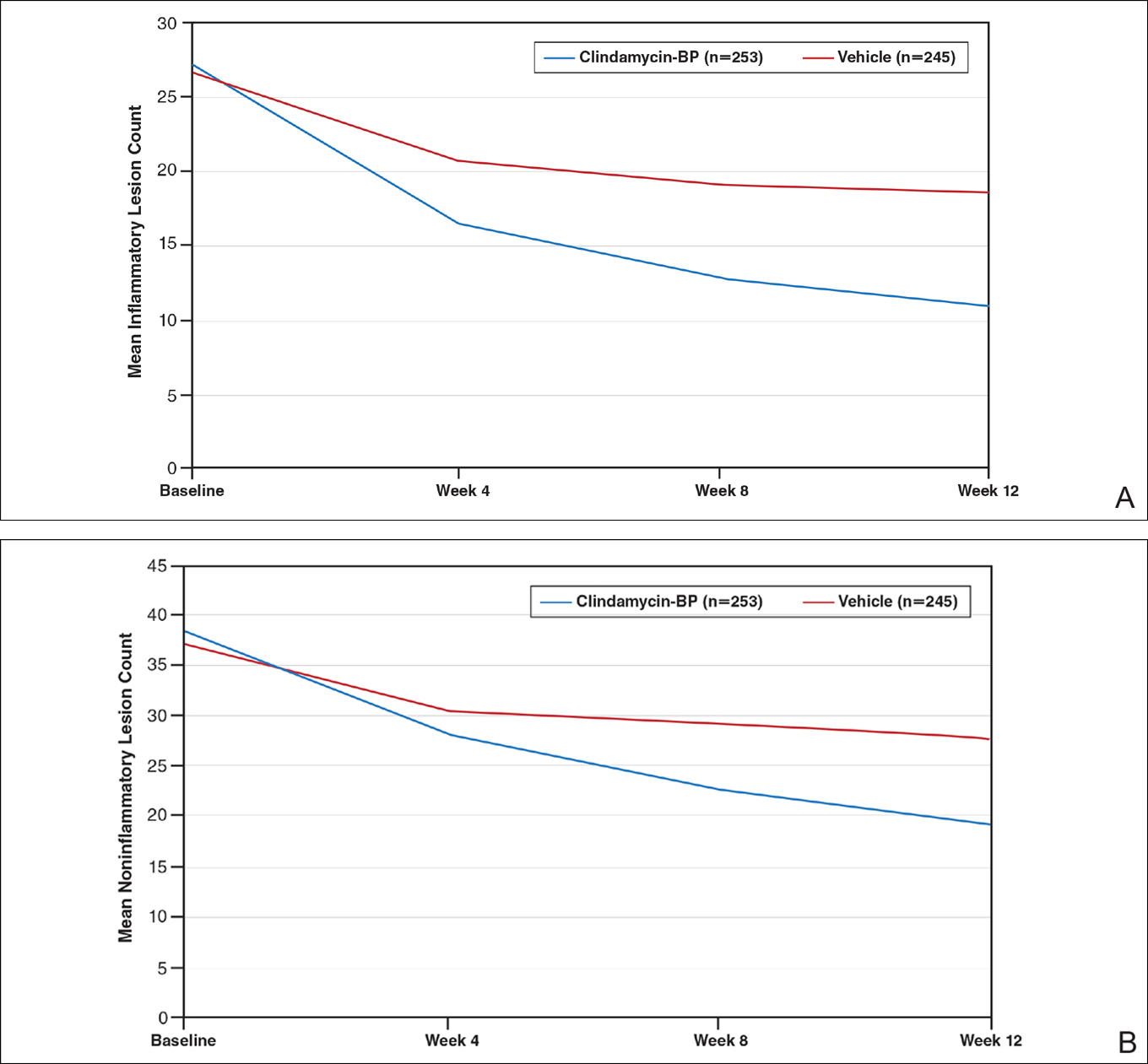
After 12 weeks of daily treatment, inflammatory and noninflammatory lesion counts decreased by a mean of 60.4% and 51.8%, respectively, with clindamycin-BP 3.75% gel compared to 31.3% and 27.6%, respectively, with vehicle (both P<.001). At weeks 4, 8, and 12, the difference in inflammatory and noninflammatory lesion counts for the active treatment was 17.4%, 24.8%, and 29.1%, respectively, and 8.1%, 19.8%, and 24.2%, respectively, for vehicle.10
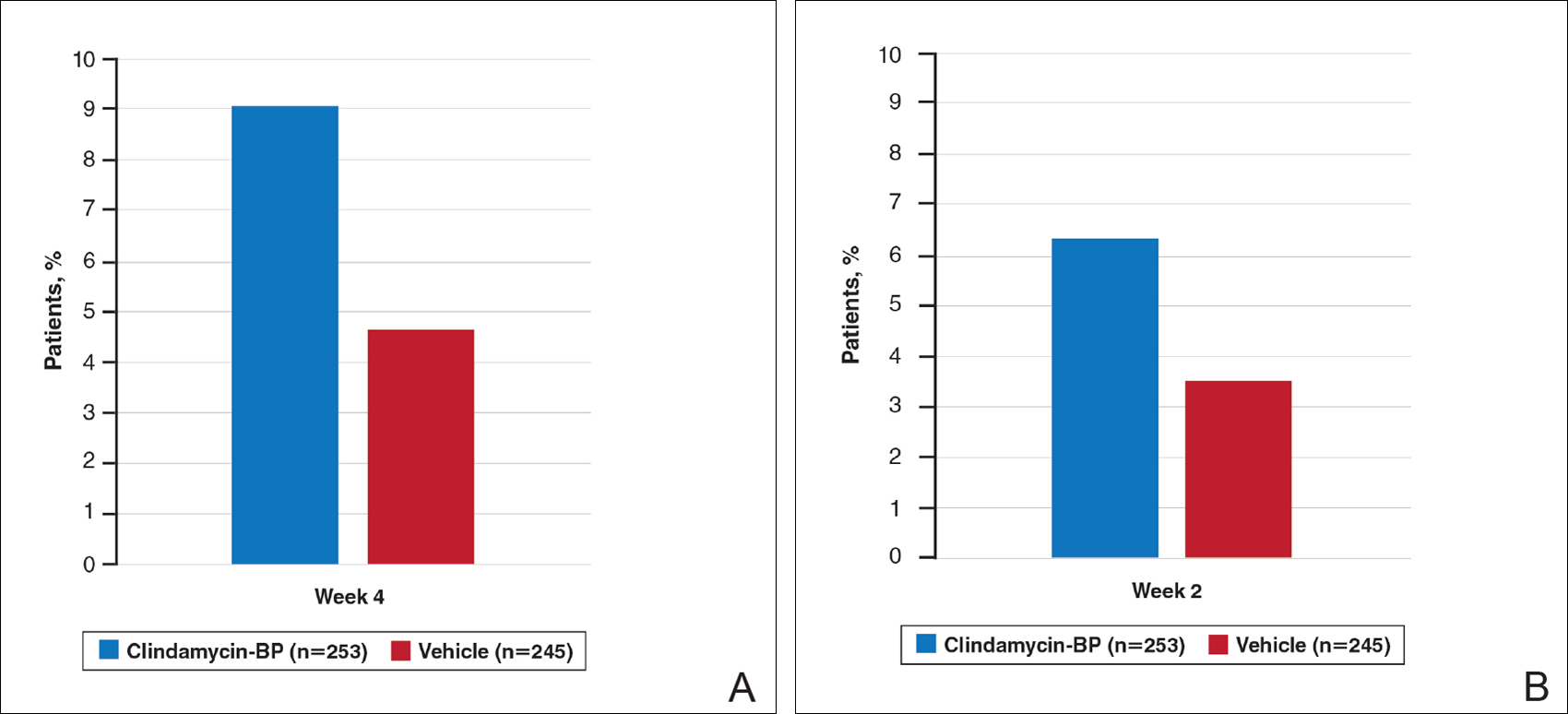
Treatment success (at least a 2-grade improvement in EGSS) was achieved by 9.1% of patients using clindamycin-BP 3.75% gel compared to 4.6% using vehicle by week 4. Additionally, 6.3% of patients considered their AV as clear or almost clear compared to 3.5% with vehicle at week 2 (Figure 1).10
This analysis represents the first attempt to evaluate and report TOA results with clindamycin-BP 3.75% gel. Time to onset of action for inflammatory lesions treated with clindamycin-BP 3.75% gel was calculated as 2.5 weeks versus 6.2 weeks for vehicle (Figure 2A). Time to onset of action for noninflammatory lesions was 3.7 weeks with clindamycin-BP 3.75% gel versus 8.6 weeks with vehicle (Figure 2B). The difference in TOA between the active and vehicle study groups was 3.7 weeks and 4.9 weeks, respectively. In addition, among actively treated patients, TOA was shorter in females (2.1 weeks) than in males (2.6 weeks) and in moderate AV (2.5 weeks) compared to severe AV (3.0 weeks).
Comment
Differences in lesion counts between clindamycin-BP 3.75% gel and vehicle suggest a clinically relevant benefit in favor of active treatment with both inflammatory and noninflammatory lesions. Nearly twice as many patients were rated as treatment successes using EGSS by week 4 or clear or almost clear as early as week 2 compared to the vehicle group.10 However, these data are suggested as an overall guide but do not provide adequate guidance on when visible improvement may start to be evident in a given patient.
The analysis reported here shows a TOA of 2.5 weeks with clindamycin-BP 3.75% gel for inflammatory lesions, approximately 4 weeks faster than with the vehicle. In most cases, a reduction in inflammatory lesions is more likely to have a greater impact on patient perception of TOA. Unless a patient is aware or focused enough to actively distinguish visibly between inflammatory and noninflammatory (comedonal) AV lesions, their eye is more likely to be drawn initially to reduction in inflammatory lesions, which are erythematous and more visible at a greater viewing distance. Although noninflammatory AV lesions usually require closer inspection to visualize them (especially closed comedones), they are often slower to respond to treatment. Analysis of the pivotal trial data reports a longer TOA with clindamycin-BP 3.75% gel for noninflammatory lesions (3.7 weeks) versus inflammatory lesions (2.5 weeks).
As expected, TOA was shorter in patients with moderate AV than severe AV (2.5 weeks vs 3.0 weeks). Time to onset of action also was shorter in females overall. It is unclear why we see gender differences in acne studies. A number of reasons have been suggested, including differences in AV pathophysiology and/or treatment adherence.11,12 Greater efficacy of clindamycin-BP 3.75% gel in females compared with males has already been reported, and better overall efficacy leading to a shorter TOA has been noted by others.13






