Stratum Corneum Absorption Kinetics of 2 Potent Topical Corticosteroid Formulations: A Pilot Study
Fluocinonide and halcinonide are class II topical corticosteroids that are indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and generally are applied to affected skin at least twice daily. This pilot study compared the absorption kinetics of cream formulations of fluocinonide and halcinonide in the stratum corneum within a 9-hour period following application. A dermal tape-stripping protocol was used to quantify corticosteroid concentration at 6 sequential depths in the skin of 4 sites on the forearm. Halcinonide and fluocinonide were extracted from the strips and concentrations were measured using liquid chromatography–mass spectrometry. Results demonstrated the immediate absorption of fluocinonide and halcinonide into the stratum corneum within 1 hour of application followed by a sustained release of halcinonide and a steady decline of fluocinonide after peaking.
Practice Points
- Fluocinonide concentration in the stratum corneum peaks within the first hour of application and then begins a steady decline.
- Halcinonide concentration also peaks within the first hour of application and remains elevated for 6 hours after application.
- Halcinonide, rather than fluocinonide, may provide clinical benefits in between doses because of its sustained release hours after application.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
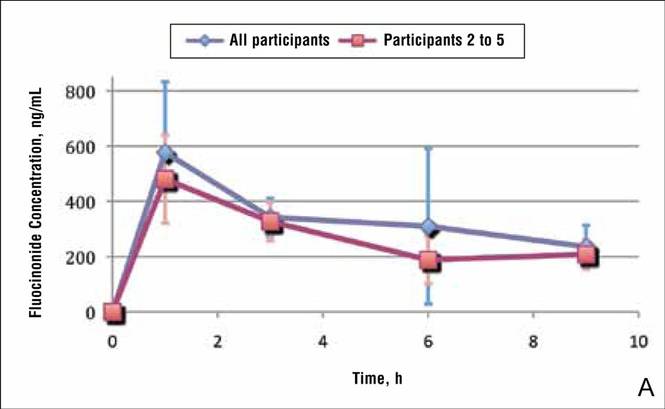
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
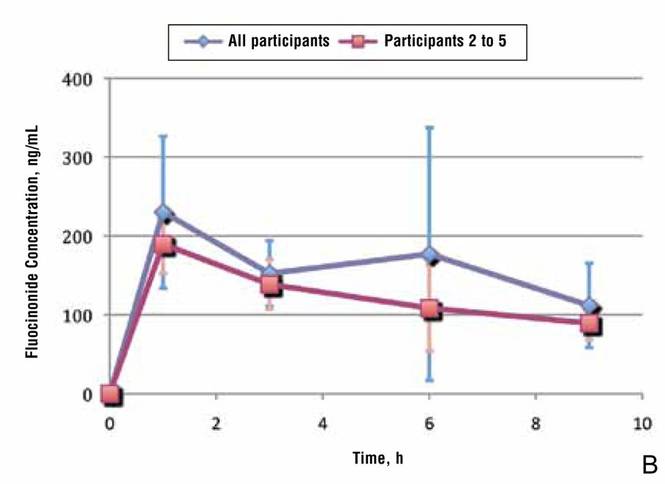
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
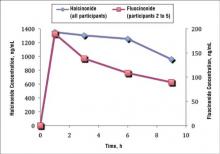
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.