A Phase 3 Randomized, Double-blind, Vehicle-Controlled Trial of Azelaic Acid Foam 15% in the Treatment of Papulopustular Rosacea
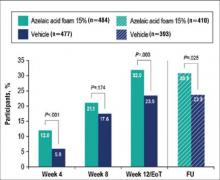
Rosacea is a chronic relapsing skin disorder primarily affecting the face. Although its etiology is not well defined, rosacea is associated with immune dysregulation and inflammation potentiated by external factors. These manifestations lead to skin sensitivity and impaired quality of life. Azelaic acid (AzA) is approved for the treatment of rosacea in a 15% gel formulation. This phase 3 study evaluated the efficacy and safety of AzA in a 15% foam formulation for the treatment of papulopustular rosacea (PPR). Coprimary efficacy end points were treatment success according to investigator global assessment (IGA) and the nominal change in inflammatory lesion count (ILC) from baseline to the end of treatment (EoT). Adverse events (AEs) were evaluated as a measure of safety. The IGA success rate at EoT was significantly greater in the AzA foam group versus vehicle (P<.001; Cochran-Mantel-Haenszel test). Likewise, nominal ILC change at EoT in the AzA foam group showed a significantly greater decrease versus vehicle (P<.001; F test). Drug-related AEs were mainly mild to moderate, cutaneous, and local. Overall, the study results support the efficacy and safety of twice-daily AzA foam 15% in patients with PPR.
APPENDIX
Supplementary Methods
Supplementary Study Design
This study met all local legal and regulatory requirements and was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the start of the study and implementation, the protocol and all amendments were approved by the appropriate independent ethics committee or institutional review board at each study site. Two protocol amendments were implemented before the first participant visit.
Exclusion criteria included the presence of dermatoses that could interfere with rosacea diagnosis or evaluation, facial laser surgery or topical use of any medication to treat rosacea within 6 weeks before randomization, systemic use of any medications to treat rosacea, and known unresponsiveness to AzA treatment. Further standard exclusion criteria included alcohol or drug use or parallel participation in other clinical studies, which were necessary to exclude undue influence on study evaluations and/or participant safety. The study was conducted by qualified investigators at 48 centers in the United States.
The investigational product was filled in identical containers according to the randomization list generated by a computer program using blocks. Complete blocks of study medication were distributed to the centers. Eligible participants were randomized 1:1 into either AzA foam or vehicle treatment groups by assignment of a randomization number at baseline. A blind investigational product under the same randomization number was dispensed to and returned from participants by study personnel who were not involved in the assessments. Blinding was achieved by using labels on the investigational products that did not allow identification of the true medication.
Compliance was evaluated from participant diaries as well as the number of expected doses and actually applied doses.
Additional Efficacy Evaluations
A number of secondary variables (not reported here) were assessed, including changes in other manifestations of PPR, as well as participant assessments of treatment response, tolerability, cosmetic preferences, and quality of life.
Additional Safety
Investigators reported a yes or no response as to whether there was a reasonable causal relationship between AEs and treatment. Moreover, AEs that began at the start of or during treatment were considered treatment emergent. Cutaneous AEs were further assessed regarding location and duration. An AE was deemed local if it occurred at the application site and transient if it subsided within 60 minutes of onset.
Statistical Analysis
The primary efficacy analyses presented here were based on the full analysis set of participants who were randomized and had medication dispensed. For participants with no EoT value, the last nonmissing value was used including baseline (last-observation-carried-forward methodology). Participants who discontinued treatment prematurely because of lack of efficacy were considered to be treatment failures, regardless of the reported IGA score. Statistical significance was needed for both coprimary efficacy variables at a 1-sided 2.5% significance level to show confirmed superiority of AzA foam versus vehicle.
A number of sensitivity analyses were performed, including an analysis of the coprimary end points using observed data, analysis of the per-protocol population of participants who did not prematurely discontinue treatment and had no major protocol deviations, subgroup analyses, and the use of statistical methods to investigate the effect of missing observations. Analyses of success rate and nominal change in ILC were repeated for each postbaseline visit using χ² and t tests, respectively. All summary and statistical analyses were performed according to the study protocol (unchanged after the start of the study) using SAS version 9.2.
Results from a prior study provided the basis for the sample size, which was calculated to show a significant difference in both primary efficacy end points with a power of 90%.8 To allow for dropouts, 480 participants in each treatment group were to be randomized for a total of 960 participants.