Biofeedback in the treatment of epilepsy
ABSTRACT
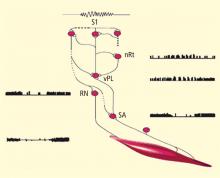
This review traces the application of electroencephalographic (EEG) operant conditioning, or biofeedback, from animal research to its emergence as an alternative treatment for the major types of seizure disorder. Initial animal studies focusing on brain mechanisms that mediate learned behavioral inhibition revealed a uniquely correlated 12- to 15-Hz EEG rhythm localized to sensorimotor cortex. We labeled this the sensorimotor rhythm, or SMR. The similarity of the SMR to the known EEG spindle pattern during quiet sleep led to the novel idea of attempting to increase the SMR using EEG operant conditioning. The hypothesis was that this might produce a corresponding increase in sleep spindle activity, thus establishing a common EEG marker for the state of motor inhibition. Results supported this hypothesis but led also to the accidental discovery of an anticonvulsant effect on drug-induced seizures in cats and monkeys. Continuing animal studies identified a pattern of neurophysiologic responses correlated with the SMR in primary motor pathways. These and other findings were indicative of reduced motor excitability. Simultaneously, we undertook studies in human epileptic subjects that documented a significant reduction in seizure incidence and severity, together with EEG pattern normalization. This work expanded internationally, resulting in numerous well-controlled group and single-case studies summarized in recent meta-analyses. Exciting new findings in functional neuroimaging/EEG correlation studies provide a rational model for the basis of these clinical effects. In recognition of the diversity of clinical applications of EEG biofeedback and the complexity of seizure disorders, this review also details specific methods used in our EEG biofeedback program.
Platform for a dual research approach
These two studies provided several interesting conclusions that directed our subsequent scientific efforts. First, in the cat study we observed a common prodrome in both SMR-trained and control animals even though the SMR-trained animals had acquired protection against seizures. This suggested a direct effect on the seizure process and not on MMH toxicity in general. Second, in our human epileptic patient, the seizures that were suppressed arose out of the unconscious state of sleep, a fact that eliminated the possibility of any voluntary countermeasure and again indicated a direct effect on the seizure mechanism. Accordingly, we undertook a dual approach to understanding the basis of this effect, involving both additional animal electrophysiologic and human clinical studies.
Animal studies evaluated motor behavior, motor reflexes, motor and thalamic unit firing, and somatosensory pathway correlates of the SMR response. Clinical studies, as reviewed in the following section, sought to further document the anticonvulsant effects of SMR operant conditioning and examine this effect on various seizure types. Possible alternative explanations, such as altered medication compliance and placebo effects, were also addressed in several comprehensive studies. Additionally, by this time other laboratories were beginning to add to the research literature in this new field.
CLINICAL STUDIES
A series of human studies followed our initial clinical report, including group studies involving crossover and placebo-controlled designs. These studies consistently reported significant seizure reductions in epileptic patients in response to reward for increasing sensorimotor EEG rhythmic activity.
Two independent meta-analyses of the peer-reviewed papers in this literature have appeared in the last decade.8,10 In a review of 24 studies involving 243 patients with predominantly partial complex seizures provided with central cortical SMR feedback training, Sterman determined that 82% of these subjects registered seizure reductions greater than 50%.8 More recently, Tan and colleagues evaluated data from 63 studies and selected for comprehensive analysis 10 studies that met stringent criteria for controls and population and seizure details.10 They reported that 79% of the patients treated with SMR feedback training experienced a statistically significant reduction in seizure frequency despite a collective history of failed medication therapy.
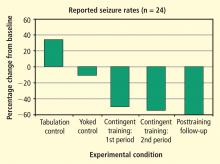
- One group simply tabulated their seizure experiences for 6 weeks using a comprehensive logging method.
- The second group received EEG feedback training for 1 hour three times a week for 6 weeks; however, the EEG signal responsible for reward was previously recorded from a different individual. This noncontingent feedback constituted a “yoked control” group.
- The third group received 6 weeks of contingent training for increasing SMR activity in somatosensory cortex while simultaneously suppressing slower 4- to 8-Hz activity.
After the initial 6 weeks, all 24 subjects were combined into one group that received 6 more weeks of contingent training only. This was followed by a 4-week period of gradual withdrawal from training and then by a final tabulation of seizure incidence during a 6-week period after training was terminated. As can be seen in Figure 5, the seizure tabulation control was associated with an increased seizure count and the “yoked control” noncontingent SMR training was associated with no significant change in seizure incidence, whereas contingent SMR training was associated with a statistically significant reduction in seizures. The statistical significance of this reduction increased progressively as subjects from the other two groups were added to a second 6-week period of contingent training, and after an additional 6 weeks following withdrawal from training. In addition to this exclusive seizure reduction after SMR contingent training, pre-/post-training neuropsychological testing showed that responding SMR-trained subjects also improved significantly in performance of tasks specific to the hemisphere contralateral to their frontotemporal lesion, indicating a reduced corrosive disturbance from the seizure focus.11