Bone density vs bone quality: What’s a clinician to do?
ABSTRACTStudies of the epidemiology of osteoporosis and of drug treatments for it have challenged the concept that denser bone means stronger bone. Bone strength or resistance to fracture is not easily measured by routine densitometry, being a function of both density and quality.
KEY POINTS
- Bone quality is a composite of properties that make bone resist fracture, such as its microarchitecture, accumulated microscopic damage, the quality of collagen, mineral crystal size, and bone turnover.
- The T score was derived from a population of white women in their mid to late 60s and older; in other populations, low T scores do not necessarily reflect the disease state—osteoporosis—with its inherent decreased strength and propensity to fracture.
- In assessing the risk of fractures, clinicians should consider not only the bone mineral density but also clinical risk factors.
- Markers of bone turnover are elevated in some cases of primary osteoporosis and return to normal levels with antiresorptive therapy but not with anabolic therapy.
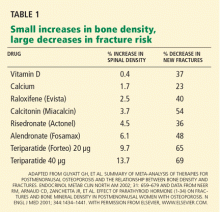
CHANGES IN DENSITY ACCOUNT FOR ONLY PART OF THE DECREASE IN RISK
BONES BECOME STRONGER BEFORE THEY BECOME DENSER
In a number of clinical trials, antiresorptive drugs of various classes started to reduce the risk of fractures before the increases in bone density reached their maximum. Raloxifene significantly reduces the incidence of fractures within 6 to 12 months of starting treatment, whereas the maximal increase in spinal bone density of 2% to 3% is seen at 3 years.15 This type of information further supported the discordance of density and bone strength and underscored the concept that drug therapy affects other factors in bone physiology.
One of these other factors is skeletal turnover, which is assessed by measuring the levels of enzymes or collagen fragments released by osteoblasts or osteoclasts in the blood or urine. These substances are markers of bone metabolism. They do not establish the diagnosis of specific diseases, but their concentrations are higher in high-bone-turnover states such as in some cases of primary osteoporosis. The topic has been reviewed in detail by Singer and Eyre.16
Antiresorptive therapy decreases the levels of these markers to normal within weeks of starting therapy. This prompt response is believed to be the reason that fracture risk reduction is seen so early. This effect of therapy represents a reduction in high osteoclastic activity and, secondarily, preservation of the microarchitecture. Meanwhile, osteoblastic activity adds bone to these less-active osteoclastic sites. If the amount is sufficient, bone densitometry may detect it.
LACK OF CHANGE IN DENSITY DOES NOT NECESSARILY MEAN LACK OF RESPONSE
The lack of change in bone density in patients taking bisphosphonates does not necessarily mean a lack of response. The following clinical scenario exemplifies this paradox.
A middle-aged woman on bisphosphonate therapy
A 68-year-old woman is seen because she seems to be having a poor response to oral bisphosphonate therapy, which was started 3 years ago after she had two vertebral fractures. Her bone density has not changed during this time, but the levels of her bone turnover markers have decreased and remain normal.
Should she start another type of therapy?
Bone turnover markers indicate a response
Studies show that patients with osteoporosis can be stratified into those at low or high risk of fractures on the basis of the activity of bone turnover markers. The risk of fractures is two times higher in people who have high levels of these markers than in those with normal levels, and can rise to four to five times as high in people who have both high marker levels and low bone density.17
All antiresorptive treatments lower the levels of these markers to the normal range and keep them low. In the patient described above, her normal levels of bone turnover markers after treatment indicate a good therapeutic response. The treatment should be continued.
WHAT’S A CLINICIAN TO DO?
These cases illustrate some important questions that often arise in the treatment of patients.
How should the risk of fractures be assessed? Bone densitometry is a better marker of fracture risk than of bone strength because it cannot detect the important qualitative elements of strength. The higher prevalence of osteoporosis in the older population gives the T score cutoff of 2.5 standard deviations below the mean a greater predictive power to diagnose osteoporosis than it does in a younger population with a lower disease prevalence. In younger patients, this cutoff at best represents low bone density and is not diagnostic of osteoporosis unless it is present with other risk factors for fracture.
Newer tools for assessing fracture risk are now entering clinical practice. Estimates of absolute fracture risk are being used,18–20 and a fracture risk assessment tool is being implemented worldwide.21–23 Developed by the World Health Organization and called FRAX, it is based on the bone mineral density of the femoral neck combined with other factors: the patient’s age, sex, weight, and height, whether the patient has a personal or family history of fracture, and whether the patient smokes, uses glucocorticoids, has rheumatoid arthritis, has secondary osteoporosis, or consumes alcohol in excess. It is available online (www.shef.ac.uk/FRAX/tool.jsp) and gives an estimate of the 10-year risk of fracture.
How should response to therapy be assessed? In clinical practice, patients who show no changes in bone density may still be responding to therapy, and the response can be detected by the levels of bone turnover markers. Patients using antiresorptive drugs have normal levels of these markers, decreased from a higher baseline value. Patients using anabolic agents show higher levels of these bone markers, indicating enhanced bone building. So therapeutic efficacy is seen as stable or increased bone density coupled with decreased and normal turnover markers with antiresorptive drug use and increased turnover markers with anabolic drug use.
When fractures occur in patients on therapy, however, it becomes difficult to assess good or poor drug response. Patients who have a fracture within the first year of therapy are best left on the treatment, since this may not generate the full response. Patients who start having fractures years into therapy, however, may be experiencing secondary forms of osteoporosis superimposed on the original primary disease.24 Vitamin D deficiency, hyperparathyroidism, and celiac disease are common problems. Or, perhaps, patients may not be adherent to therapy.25–27 Poor compliance, inappropriate use of medications (especially the bisphosphonate drugs), or even problems of malabsorption of oral medication may be a consideration. The intravenous forms of bisphosphonate drugs warrant consideration in this scenario.28–30
In the future, we may have better tests of bone quality. One such test, called finite element analysis, uses computer modeling and three-dimensional imaging. It has been used for years by engineers designing and testing the strength of bridges, airplanes, and other structures and is now being evaluated as a way to estimate bone strength.
In summary, bone physiology and bone strength are very complex issues that have recently attained new and important nuances. The original use of bone densitometry was to assess the risk of fragility fractures and, secondarily, to diagnose primary osteoporosis in the population of patients for which it was originally developed. While the bone densitometry score does bear some relationship to bone strength, it is not a sufficient surrogate marker in many cases. Hence, clinicians need to judiciously use these testing procedures in combination with a number of clinical factors to diagnose osteoporosis and assess the response to therapy.