Using biochemical markers of bone turnover in clinical practice
ABSTRACTBiochemical markers of bone turnover provide clinically useful evidence of the normal and pathologic processes that reflect bone cell activity in the skeleton. Understanding the behavior of markers of bone formation and bone resorption should aid in managing patients with a variety of skeletal disorders.
KEY POINTS
- Biomarkers of bone formation and resorption reflect the overall osteoblastic and osteoclastic activity in the skeleton and in some situations may serve as surrogates for histologic examination of bone.
- Biomarkers of bone turnover can be used to document the effects of therapeutic agents in some patients with osteoporosis and possibly reduce the need for frequent bone density testing.
- In cancer patients with bone metastases, biomarkers of bone resorption provide evidence of the efficacy of antiresorptive therapy. The baseline levels also have prognostic value: patients with the highest levels have the worst prognosis.
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
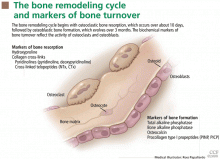
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.