Sleep apnea and the heart
Release date: September 1, 2019
Expiration date: August 31, 2022
Estimated time of completion: 0.75 hour
Click here to start this CME activity.
ABSTRACT
The normal sleep-wake cycle is characterized by diurnal variations in blood pressure, heart rate, and cardiac events. Sleep apnea disrupts the normal sleep-heart interaction, and the pathophysiology varies for obstructive sleep apnea (OSA) and central sleep apnea (CSA). Associations exist between sleep-disordered breathing (which encompasses both OSA and CSA) and heart failure, atrial fibrillation, stroke, coronary artery disease, and cardiovascular mortality. Treatment options include positive airway pressure as well as adaptive servo-ventilation and phrenic nerve stimulation for CSA. Treatment improves blood pressure, quality of life, and sleepiness, the last particularly in those at risk for cardiovascular disease. Results from clinical trials are not definitive in terms of hard cardiovascular outcomes.
KEY POINTS
- Diurnal variations in blood pressure, heart rate, and cardiac events occur during normal sleep.
- While normal sleep may be cardioprotective, sleep apnea disrupts the normal sleep-heart interaction.
- Untreated severe sleep apnea increases the risk for cardiovascular events.
- Treatment with continuous positive airway pressure (CPAP) may reduce the risk of cardiac events based on some data, though randomized studies suggest no improvement in cardiovascular mortality.
- Poor patient adherence to CPAP makes it difficult to evaluate the efficacy of CPAP treatment in clinical trials.
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
,Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
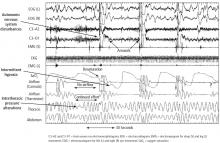
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.