Community-Acquired Pneumonia: Treatment
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
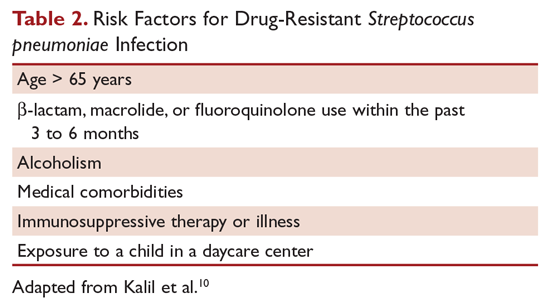
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
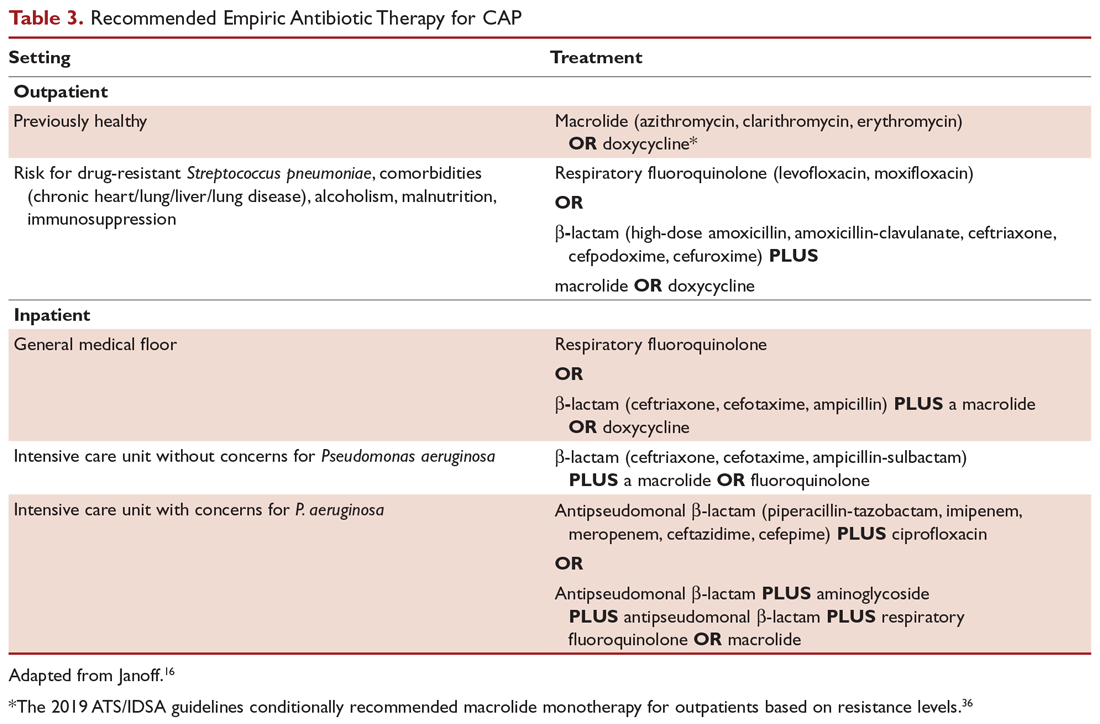
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19






