Fetal pulse oximetry: 8 vital questions
Will this noninvasive technique improve assessment of fetal well-being? The authors analyze what the evidence to date does and does not tell.
Question 6Will it improve neonatal outcomes?
Neonatal outcome is the ultimate endpoint in obstetrical care. In the randomized trial by Garite et al,3 there was no difference in neonatal outcome between the groups using or not using fetal pulse oximetry. According to Chua et al,33 FSpO2 levels measured even 10 minutes before delivery have no relation to neonatal outcome.
Leszczynska-Gorzelak et al22 believe FSpO2 is more predictive of neonatal outcome in the first stage than the second. However, Apgar score had no relationship with FSpO2readings in the first or second stage. Butterwegge34 reported 6 cases of FSpO2 below 30% for more than 30 minutes, all with good neonatal outcome, and Alshimmiri et al23 noted that only normal FSpO2 correlates with fetal well-being. Thus, it appears that the NPV of fetal oximetry is of greater practical value than other attributes.
Question 7How precise is it?
The Nellcor system monitors the quality of FSpO2 measurement; no value is displayed if the signal lacks the characteristics of a fetal arterial plethysmographic curve or if contact between sensor and skin is insufficient. Because of fetal movements and other artifacts, posting time is always less than 100%.
In the French multicenter study,25 the mean reliable signal time in the first stage of labor was only 64.7%—even less in the second stage (54%). Signal retention was 67% in the randomized trial by Garite et al. 3
Many artifacts may impede signal acquisition and impact the reliability of a reading:
- The sensor’s position on the fetal head. For example, the difference in FSpO2 readings between the forehead and occiput may be as much as 13.4%. (The sensor is designed to go against the fetal cheek, but may move around.)
- Incomplete sensor-to-skin contact, such as with high fetal head station, -2 or above.
- Marked caput formation.
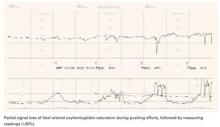
- Increased intrauterine pressure accompanying contractions, especially at presentation stations of +2 or below (FIGURE 2). FSpO2 monitoring requires detection of fetal pulses, which may be undetectable when the surrounding pressure is high, resulting in a loss of signal.
- Interposition of vernix or fetal hair.
- Presence of meconium, which behaves like a red-light filter, altering the ratio of red to infrared light and resulting in artificially low values.35 This theoretical concern is rejected by Yam et al,36 who did not observe any effect of meconium on FSpO2 values. (When the amniotic fluid is meconium-stained, Carbonne et al37 showed that fetal oximetry is a better predictor of meconium aspiration syndrome than FSB sampling.) The data on the influence of meconium on FSpO2 readings remain contradictory.
All these conditions may impair precision and contribute to poor sensitivity.
FIGURE 2 A weakening signal during pushing
Question 8Is it easy to use?
An Australian survey38 assessed clinicians’ perceptions during placement of the oximetry sensor. Ease of placement was rated as good or excellent in 71% of cases, and the patient’s comfort was rated as good or excellent in 90% of cases. Chua et al39 reported a mean insertion time of 90 seconds, with a reliable signal obtained within 5 minutes in 87% of placements. The French multicenter study25 mentioned earlier concluded that the procedure is satisfactory and easier than FSB sampling. The device itself was harmless to both mother and fetus.40
Potential research directions
Fetal pulse oximetry may be an effective tool in clinical scenarios such as:
- fetal arrhythmias with uninterpretable FHR tracing
- fetal tachycardia associated with maternal fever, thyrotoxicosis, or fetal supraventricular tachycardia, when distinguishing other contributions to tachycardia may be difficult
- fetal bradycardia caused by a complete heart block, which may render EFM undecipherable
- when amnioinfusion is attempted for variable decelerations and it is necessary to differentiate a nonreassuring FHR tracing related to transient in utero stress (eg, umbilical cord compressions) from ominous tracings
Another area not yet addressed is cost-effectiveness beyond the immediate direct costs (approximately $11,000 for the monitor and $150 for each disposable sensor). Also uncertain is whether laboring women will accept the device (how disturbing or invasive it is perceived to be) and how acceptable or applicable it is outside tertiary institutions.
Dr. Vidaeff reports no financial relationships relevant to this article. Dr. Ramin reports grant support from the US National Institutes of Health.