2014 Update on minimally invasive gynecology
The cesarean scar defect: A common etiology of abnormal uterine bleeding
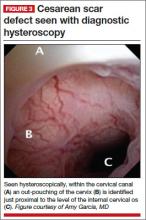
CESAREAN SCAR DEFECT DIAGNOSED WITH HYSTEROSCOPY
![]()
![]()
Videos courtesy of Amy Garcia, MD
Related article: Dr. Garcia discusses the FIGO classification and the PALM-COEIN pneumonic in Update: Minimally invasive gynecology (April 2013)
![]()
![]()
A pair of studies shed light on CSD
Two recent European publications by van der Voet and colleagues addressed CSD and its association with AUB. These studies refer to CSD as the “niche” within the cesarean scar, but for the purpose of this article, I will use the term CSD. The first is a prospective cohort study, in which the authors addressed the definition, diagnosis, and prevalence of a defect within the cesarean scar and reported the incidence of associated AUB.7 The second publication is a systematic review which includes a critical investigation of minimally invasive therapy for CSD-related AUB.8 Both publications provide current clinical insight into the evaluation and management of AUB associated with CSD.
Related articles:
• Update on abnormal uterine bleeding Malcolm G. Munro, MD (March 2014)
• Update on Technology Barbara S. Levy, MD (September 2013)
• STOP performing dilation and curettage for the evaluation of abnormal uterine bleeding Amy Garcia, MD (Stop/Start, June 2013)
THE NICHE IN THE SCAR
van der Voet LF, Bij de Vaate AM, Veersema S, Brolmann HAM, Huirne JAF. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244.
Most studies reporting the prevalence of cesarean delivery–associated postmenstrual bleeding are based on populations of women who were symptomatic with AUB, thus infusing a potential referral bias into these prevalence estimates. In contrast, this study by van der Voet and colleagues utilizes a prospective cohort design, making it the only study to date to enroll a random cohort of patients immediately after having undergone cesarean delivery.
Details of the study
The purpose of the study was to evaluate the prevalence of CSD formation in the cesarean scar at 6 to 12 weeks after cesarean delivery with TVUS and gel infusion study (GIS) in 197 women. The uterus was closed in two layers for four women and in one layer for all others.
The cohort was followed with menstruation questionnaires at 6 to 12 weeks, 6 months, and 12 months after surgery. The questionnaire response rate at 12 months for those women who had both TVUS and GIS evaluation of the scar was 73%. Data analysis accounted for confounding factors such as breastfeeding and amenorrhea, use of hormonal contraception, use of a levonorgestrel intrauterine system (LNG-IUS) as well as a body mass index (BMI) of at least 25 kg/m2.
Consistent with previous studies showing the superiority of saline-infused studies over TVUS for CSD identification,4 van der Voet and colleagues found that GIS was more sensitive than TVUS in diagnosing CSD (64.5% vs 49.6%, respectively). The percentage of women with CSD who had undergone two cesarean deliveries was 68.2%, while the percentage with CSD who had undergone three cesarean deliveries was 77.8%.
Data analysis correlated postmenstrual bleeding with the following CSD characteristics:
- depth and width of the defect
- residual myometrial thickness to the serosal surface of the uterus
- ratio of residual myometrium divided by the adjacent normal myometrial thickness.
Those women who had a ratio of residual myometrium to adjacent normal myometrium of less than 0.5 were more likely to report postmenstrual bleeding than those with a ratio greater than 0.5 (odds ratio, 6.1; 95% confidence interval, 1.74–21.63). The investigators stated that 1 out of 3 women with CSD identified by GIS reported postmenstrual bleeding, compared with 1 out of 10 women without identifiable CSD.
Study takeaways have merit
In summary, despite the small cohort of 197 women and the relatively short observation period of 1 year, these data collected by van der Voet and colleagues enable the gynecologist to begin to more fully understand the potential impact of cesarean section and the probability of AUB following an abdominal delivery. Applying these study statistics to the number of cesarean sections performed annually in the United States translates to nearly 280,000 women yearly who may experience postmenstrual bleeding related to a defect in the cesarean section scar.
Prospective cohort studies with longer follow-up periods are needed to assess the longer-term risks of CSD-related bleeding. As the authors suggest, perhaps the possibility of post–cesarean section AUB should be considered as part of the informed consent process for cesarean delivery.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
• Contrast infusion sonography has better sensitivity than TVUS at identification of the scar defect.
• About 64.5% of women are predicted to have scar defects after one cesarean delivery.
• The incidence of scar defects increases with increasing number of cesarean deliveries.
• One of three women with CSD is predicted to experience postmenstrual bleeding.
• Women with deeper and wider defects are more likely to experience postmenstrual bleeding.
• Post–cesarean section AUB is a probable occurrence in approximately 20% of all cesarean deliveries. Perhaps this information should be considered part of the informed consent process for cesarean delivery.