New group B strep guidelines clarify management of key groups
The CDC’s latest recommendations, endorsed by ACOG, spell out screening and intrapartum prophylaxis among women who experience preterm labor, preterm premature rupture of membranes, group B Streptococcus in urine, and allergy to penicillin
IN THIS ARTICLE
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.
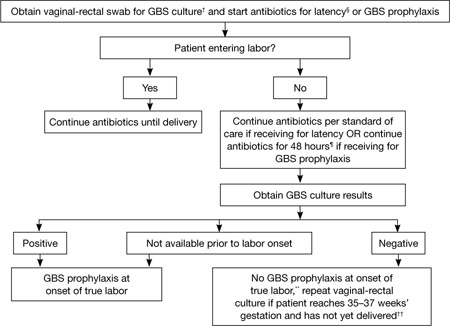
FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."






