Effectiveness of duloxetine in treatment of painful chemotherapy-induced peripheral neuropathy: a systematic review
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life. To date, the therapeutic options for CIPN are limited. We performed a systematic literature search of the PubMed and Scopus databases to assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN. The search included randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in treatment of CIPN. A descriptive analysis of the studies was performed. The PubMed database online search identified 41 publications, and a second database search through Scopus identified 29 publications. A total of 10 full-text articles were assessed for eligibility, with 5 articles excluded. Altogether, the included studies reported 431 patients with painful CIPN. An improvement in pain scores was the primary and/or secondary endpoint in the included studies. Pain was assessed by 6 different scores. Comparator drugs were used in 4 studies in our review. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity therapy. The chemotherapeutic agents used in the studies were the following: paclitaxel (52.9%), oxaliplatin (39.7%), R-CHOP (rituximab, doxorubicin, vincristine, and cyclophosphamide; 3.30%), combined bortezomib-dexamethasone (1.89%), FOLFOX (folinic acid, fluorouracil, and oxaliplatin; 1.18%), and other taxanes (0.94%). From the descriptive analyses, and from the available data of relatively small sample sized studies, it can be concluded that despite the above limitations, duloxetine remains a useful therapeutic option for pain in CIPN patients, regardless of the type of chemotherapeutic agent used.
Accepted for publication November 20, 2018
Correspondence Wael Ibrahim, MD; dr.wael_ezzat@hotmail.com
Disclosures The authors report no disclosures/conflicts of interest.
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0436
Study characteristics
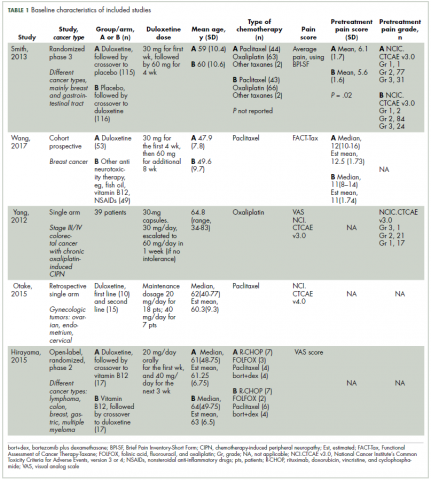
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
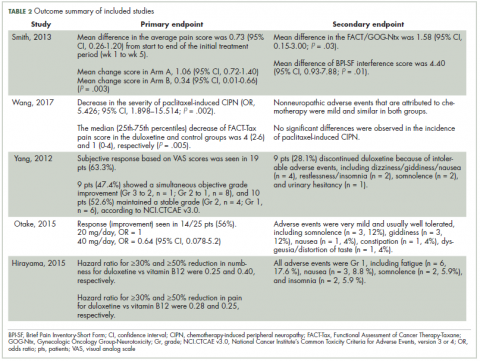
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.