Gastrointestinal cancers: new standards of care from landmark trials
Received for publication October 2, 2017
Published online ahead of print March 28, 2018
Correspondence
David H Henry, MD; David.Henry@uphs.upenn.edu;
Daniel G Haller, MD, FACP, FRCP; Daniel.Haller@uphs.upenn.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2018; 16(2): e117-e122
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0377
Listen to the audio here
Submit a paper here
More importantly, as we’re seeing with Jimmy Carter with checkpoint treatment (for melanoma that had metastasized to the brain), responses lasted for more than 6 months in about two-thirds of patients, even a complete response, so this is just off the wall. I mean, this is not what you would expect with almost any other treatment. The data are the same for atezolizumab and for pembrolizumab. What seems to be true is that in the GI tumors and colon cancer, MSI-high seems more important than expression of PD-1 or PD-L1 (programmed cell death protein-1 or programmed cell death protein-ligand 1).
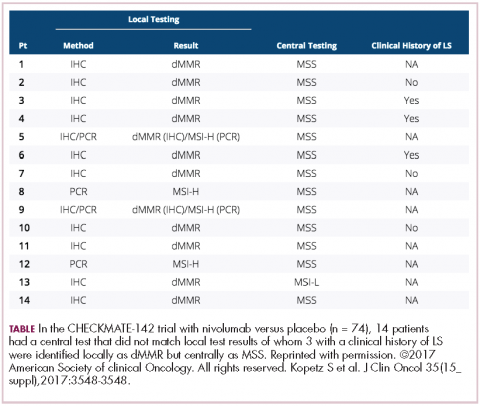
In different tumor sites, PD-1 or PD-L1 measurement may be important, but in these tumors, and in colorectal cancer, it looks as if MSI-high is the preferred measurement. Recently ASCO, together with the American Society for Clinical Pathology, College of American Pathologists, and Association for Molecular Pathology, came out with guidelines on what you should measure in colorectal cancer specimens. Obviously, one is extended RAS. They say you should get BRAF for prognosis, but it may also be a prognostic factor that leads you to treat, which ultimately makes it a predictive factor, so the data from Kopetz might suggest that will move up to something you also must measure. If patients have the BRAF mutation, it’s important they know that it’s a poor prognostic sign. But if they come in with literature saying they might live 36 months when their actual outcome is about a third of that, you need to frame your discussion in that regard and make sure they understand it.
,The guidelines also suggested getting MSI-high, and certainly prognostically in early-stage disease, but now it’s going to be a predictive factor, so in the month in which these recommendations are made, two of them are already out of date. They also didn’t include human epidermal growth factor receptor 2 (HER2), and what we’ve heard from the HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) trial is that for those patients who got the trastuzumab and pertuzumab combination – and this is another 5% of patients – almost the same data was seen as in the MSI-high patients with checkpoint inhibitors. That is double-digit response rates and durable responses. As I said, we’re very much nearing in colorectal cancer what’s now being done in non-small cell lung cancer.
DR HENRY Indeed. Could you comment on the BILCAP study and adjuvant capecitabine for biliary tract cancer?
DR HALLER There are large meta-analyses looking at adjuvant therapy for biliary tract cancers typically from fairly small, fairly old studies that all suggest that in certain stages of resected biliary tumors, either bile duct or gall bladder, adjuvant treatment works, and typically either chemotherapy and radiotherapy, or chemotherapy alone, but not radiotherapy alone.
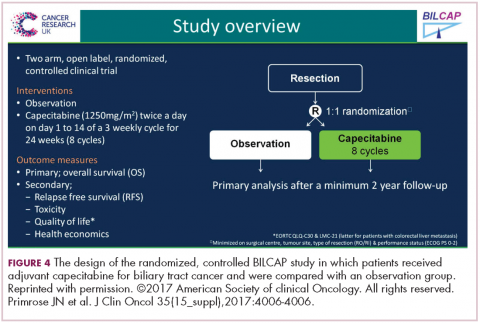
Capecitabine has been used for metastatic disease for years, mostly by default, and because most GI tumors have some response to fluoropyridines. But we’re finally able now to do large trials in biliary tumors, so this trial was a very large study with almost 450 patients from the United Kingdom over an 8-year period. About 20% were gallbladder, so the R0 surgery was about 60%, R1 at about 40% (Figure 4).6
The endpoint of the study was survival advantage, and when they did the protocol analysis, the survival for the treated population was 53 months and for the observation arm, 36 months, so that was a hazard ratio of 0.75, which is acceptable in an adjuvant study. It’s simple drug to give, and usually tolerable, so this will represent a new standard of care. Of course, in the advanced disease setting, the gemcitabine–cisplatin combination is the standard of care for metastatic disease. It’s a little more toxic combination, but we know that’s standard. There’s an ongoing study in Europe called the ACTICCA-1 trial, and this is gemcitabine–cisplatin for 6 months versus not capecitabine, but a control arm. My guess is if the capecitabine study was positive, that this also will be a positive trial, because gemcitabine–cisplatin is probably more active. Then, we’ll have 2 standards, and I don’t think anyone is going to compare capecitabine with gemcitabine–cisplatin.
What you’ll have are two regimens for two different populations of patients. Perhaps for the elderly and people who have renal problems, capecitabine alone will give them benefit, and then you’ll have gemcitabine–cisplatin, which may be just a more toxic regimen, but also more effective for the younger, healthier people with fewer comorbidities.