Prevention and treatment options for mTOR inhibitor-associated stomatitis
Inhibitors of mammalian target of rapamycin (mTOR) are approved for treatment of various advanced solid tumors (renal cell carcinoma, neuroendocrine tumors, breast cancer). mTOR inhibitor-associated stomatitis (mIAS), a frequent, early-onset side effect of this drug class, can be dose limiting and diminish patient quality of life. This systematic review describes clinical presentation and pathophysiology of mIAS and reviews its prevention and treatment. Published literature on mTOR inhibitors and their side effects, and their prevention and treatment were reviewed. Preventative and management strategies under evaluation in clinical trials were also reviewed. The majority of patients develop a mild form of stomatitis that does not interfere with treatment of their disease. A minority of patients can develop moderate to severe mIAS that can be managed with treatment modification or discontinuation, but these approaches may have an impact on disease outcome. mIAS is a relatively recent phenomenon, so evidence-based preventive and therapeutic measures are not yet available – although under active investigation – and current management is based largely on collective experience with chemotherapy- or radiation-induced oral mucositis and aphthous ulcers. Expert opinion and clinical experience from managing oral mucositis and apthous ulcers suggest that management of mIAS should focus on three major approaches: prevention, early aggressive treatment, and, when needed, more aggressive pain management. Early recognition and diagnosis of mIAS facilitate early intervention to limit potential sequelae of mIAS and minimize the need for mTOR inhibitor dose reduction and interruption. Funding for manuscript development was provided by Novartis Pharmaceuticals Corporation.
Accepted for publication February 17, 2017
Correspondence Kavitha J Ramchandran, MD; kavitha@stanford.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(2):74-81
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0335
Submit a paper here
Mammalian target of rapamycin (mTOR), a serine–threonine protein kinase, operates in the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT)–mTOR signal transduction pathway regulating both normal and cancer cellular processes, including cell growth, proliferation, motility, survival, and protein and lipid synthesis.1 Genetic alterations affecting this pathway, including mutations in receptor tyrosine kinases PI3K and AKT, occur frequently in human cancers,2 supporting the rationale to develop drugs that target pathway components, such as mTOR inhibitors.
Two mTOR inhibitors are currently approved by the US Food and Drug Administration for cancer treatment: temsirolimus, for advanced renal cell carcinoma (RCC; approved 2007)3 and everolimus, for advanced RCC (approved 2009), advanced pancreatic neuroendocrine tumors (pNET; approved 2011), and hormone receptor-positive (HR-positive), human epidermal growth factor receptor-2 (HER2)-negative advanced breast cancer (approved 2012).4 Another mTOR inhibitor, sirolimus, is approved for use as an immunosuppressive agent and prophylactic against organ rejection after kidney transplant.5
Stomatitis, inflammation of the oral mucosa with contributing factors of genetic predisposition, nutritional deficiencies, infections, and immunological or hematologic dysfunction,6 occurs frequently as a side effect associated with mTOR inhibitor treatment.7-9 Left untreated or managed unsatisfactorily, mTOR inhibitor-associated stomatitis (mIAS) may cause patients discomfort and trouble with maintaining adequate nutritional intake and proper oral hygiene, as well as strict adherence to cancer treatment. It is therefore important for health care providers of cancer patients receiving mTOR inhibitor treatment to be knowledgeable about this side effect. The purpose of the present systematic review of published literature is to provide a better understanding of the differential diagnosis of mIAS, the pathophysiology of mIAS, preventive strategies for patients initiating mTOR inhibitor treatment, and treatment options available to manage mIAS.
,Method
The PubMed database was searched with the terms mTOR inhibitor and stomatitis (no date restriction); 79 articles were retrieved, and all abstracts were reviewed to select those relevant to the aims of this review article. To understand future directions for management and prevention of mIAS, a search of clinicaltrials.gov was performed with the terms temsirolimus everolimus stomatitis yielding 12 clinical trials, of which 4 were excluded: 1 trial was terminated due to slow accrual, the status of 1 trial had not been verified in >2 years, and 2 studies focused on efficacy outcomes. A search of the American Society of Clinical Oncology (ASCO) meeting abstracts database was performed to assess the availability of clinical trial data; the search was limited to 2011-2016 and terms were stomatitis in the title and mTOR in the abstract or title. Seven abstracts were retrieved; 2 discussed stomatitis prevention (1 as a “trial-in-progress” and 1 presented results of the trial); the other 5 abstracts presented meta-analyses or reviews of previous clinical studies to assess the risk, incidence, management, and resolution of mIAS.
Review findings
Incidence of mIAS in patients treated for cancer
Two recent meta-analyses quantified the rate of mIAS in patients receiving mTOR inhibitors. Shameem and colleagues10 identified 9 randomized studies of everolimus (8 phase 3, 1 phase 2) and 2 of temsirolimus (1 each phase 2 and 3) involving a total of 4752 patients with a variety of tumor types including angiomyolipoma, breast, gastric, giant cell astrocytoma, pNET, and RCC. Patients received everolimus monotherapy (n = 1,075) or in combination with exemestane (n = 485), tamoxifen (n = 54), letrozole (n = 137), or octreotide (n = 216). Temsirolimus was administered as monotherapy (n = 208) or in combination with interferon
(n = 210) or letrozole (n = 550). The incidence of all-grade stomatitis in the 11 studies ranged from 11%-63%, and the overall incidence of any grade stomatitis was 33.5% (95% confidence interval [CI], 21.9%-47.6%). The concurrent use of a second agent may have confounded these findings because, for example, stomatitis has been reported in pooled analyses and in postmarketing experience with letrozole.11
Rugo and colleagues12 evaluated the incidence of stomatitis in 1455 patients participating in 5 phase 3 randomized clinical trials of everolimus in breast cancer, carcinoid tumor, pNET, and RCC. Patients received everolimus monotherapy
(n = 478) or in combination with exemestane (n = 482), trastuzumab plus vinorelbine (n = 280), or octreotide
(n = 215). The incidence of stomatitis in patients receiving everolimus was 59%-71%, compared with 19%-29% in 1,071 patients of the comparator arms (placebo, and placebo–trastuzumab–vinorelbine). The overall incidence of any grade stomatitis was 67%; most events were mild (grade 1/2); 9% of stomatitis events were moderate to severe (grade 3/4).
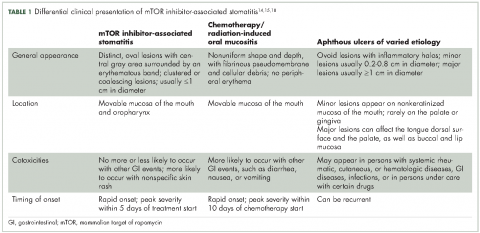
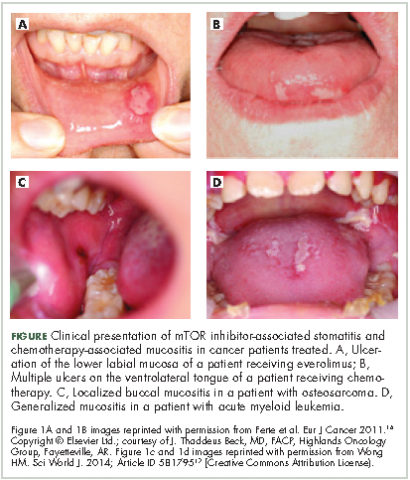
Differential clinical presentation of mIAS and severity
Oral mucositis is a common significant adverse event (AE) that occurs in patients with cancer who receive standard chemotherapy regimens and/or radiation therapy,13 so it is important to recognize that the clinical presentation of mIAS differs from that of oral mucositis (Table 1, Figure 14,15).16 mIAS shares some similarities with aphthous ulcers (also referred to as canker sores), a common oral condition with varied causes related to systemic disorders, gastrointestinal disorders, and infections, among others .17 In general, mIAS ulcers develop with a median onset of 10 days (range, 4-25 days) after initiation of mTOR inhibitor treatment and resolve in about 1-3 weeks after dose interruption/reduction of everolimus.16,18,19 mIAS ulcers appear as distinct, oval lesions with a central gray area surrounded by peripheral erythema. They are usually localized to the movable mucosa of the mouth and oropharynx. Although mIAS lesions are usually small, they are quite painful and may cluster.