Polycythemia Vera and Essential Thrombocythemia
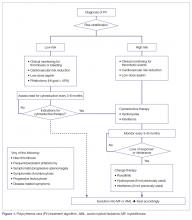
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented acquired von Willebrand syndrome, with or without extreme thrombocytosis, or in those in the very low-risk category according to the IPSET-thrombosis model [55,87]. The risk/benefit ratio of antiplatelet agents in patients with ET at different thrombotic risk levels was assessed in poor-quality studies and thus remains highly uncertain. Platelet-lowering agents are sometimes recommended in patients with low-risk disease who have platelet counts ≥ 1500 × 103/μL, due to the potential risk of acquired von Willebrand syndrome and a risk of bleeding (this would require stopping ASA) [109]. Cytoreduction may also be used in low-risk patients with progressive symptoms despite ASA, symptomatic or progressive splenomegaly, and progressive leukocytosis.
Intermediate-Risk ET
This category includes patients older than 60 years, but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in most patients [110]. In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/μL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs [64].
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (n = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events [111]. As a result of this study, hydroxyurea is often preferred to anagrelide as frontline therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (n = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate [112]. The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET and the former utilizing Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.