Clinician Telephone Training to Reduce Family Tobacco Use: Analysis of Transcribed Recordings
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
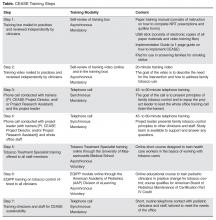
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.