Management Challenges in Sarcoidosis
Case Continued
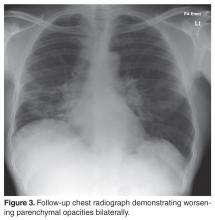
The patient was started on corticosteroid eye drops and steroid ointment for his ophthalmologic and cutaneous sarcoidosis, respectively, and symptoms gradually improved over the next few months. As the patient was symptomatic with cough and breathlessness and there was evidence of reduction in FVC (along with reduced DLco), a trial of oral corticosteroids was considered to treat the pulmonary sarcoidosis.
What is first-line pharmacological treatment for sarcoidosis and when is it indicated?
Treatment of sarcoidosis is dependent upon the severity of disease and organ involvement at the time of the diagnosis. Glucocorticoids have traditionally been considered first-line pharmacological agents in selective cases, as a significant proportion of patients do not require drug treatment due to the propensity for spontaneous remission. Furthermore, sarcoidosis remains stable without anti-inflammatory/immunosuppressive therapies in a majority of patients. A number of clinical trials have evaluated the value of corticosteroids in the management of sarcoidosis, with variable outcomes [10–16]. The disease tends to be severe in patients of African descent compared with patients from other racial backgrounds. The European cohort of sarcoidosis patients generally have milder disease with less propensity for vital organ involvement such as cardiac or central nervous system (CNS) disease.
The decision to initiate corticosteroids for sarcoidosis is not a straightforward one as there is variability in symptom presentation, disease severity, and response to corticosteroids. We initiate first-line therapy with oral prednisolone in the following circumstances:
- Evidence of pulmonary impairment (forced vital capacity FVC < 80% predicted) with or without reduction in gas transfer (DLco) along with respiratory symptoms of cough, chest pain, and/or breathlessness (as seen in the case patient)
- Vital organ involvement such as cardiac, ophthalmic (such as panuveitis) or CNS sarcoidosis once confirmed by respective investigations
- Selective cases of sarcoid-associated pulmonary hypertension (SAPH) along with close liaison with pulmonary hypertension specialists
We recommend an initial starting dose of 20 to 40 mg of prednisolone for a period of 1 to 3 months, followed by maintenance dose of 10 mg or less for a further 6 to 9 months, aiming for a total duration of treatment of 12 months. However, the duration may vary depending on the response and any associated adverse effects with corticosteroids. It is usual practice to supplement with calcium and vitamin D when beginning patients on oral corticosteroids due to the potential risk of osteoporosis. However, this may result in significant hypercalcemia, which itself may be an endocrine manifestation of sarcoidosis. Hence, we recommend monitoring serum calcium during treatment and supplement vitamin D in patients who are vitamin D–deficient [17]. Furthermore, serum vitamin 1,25(OH)2 vitamin D3 has been demonstrated to the best available test to evaluate vitamin D status in sarcoidosis [18].
Case Continued
What are the preferred pharmacological agents for second-line treatment in sarcoidosis?
Alternative immunosuppression should be considered in the following circumstances in patients diagnosed with sarcoidosis:
- Failure or less than optimal response to oral corticosteroids
- Use as a steroid-sparing agent in patients requiring high doses of steroids for symptomatic control
- Failure to tolerate corticosteroids due to significant adverse effects such as excessive weight gain, steroid-induced psychosis, osteoporosis, and worsening diabetic control
A small trial of 11 patients examined azathioprine as a steroid-sparing agent and found it as an acceptable immunosuppressive agent for that purpose [19]. It was associated with good safety profile and adherence to treatment was 82% (9 out of 11 patients). However, the small sample size makes it difficult to draw firm conclusions from the findings of this study. In another trial evaluating methotrexate as a steroid-sparing agent in the first year after the diagnosis of sarcoidosis, Baughman and colleagues [20] reported that methotrexate is an attractive alternative to other immunosuppressive agents in term of a steroid sparer. In this double-blind randomized controlled trial (RCT), 15 patients were studied with at least 6 months of treatment with methotrexate vs placebo. There was a significantly reduced dosage of prednisolone observed in methotrexate group. However, the difference was not significant when data were analyzed for all patients, including the dropouts. More recently, a large international retrospective cohort study of 200 patients with sarcoidosis demonstrated that both methotrexate and azathioprine have similar efficacy and steroid-sparing capacity in sarcoidosis [21]. However, infection rates were significantly higher in the azathioprine group as compared to methotrexate (34.6% vs. 18.1%, P = 0.01). Hence methotrexate should be considered as a preferred second-line agent in sarcoidosis after detailed discussion about potential side effects.