Management of Status Epilepticus in Adults
Pathophysiology of SE
Most seizures are self-terminating phenomena lasting from a few seconds to a few minutes [49]. One of the distinguishing characteristics of seizures evolving into SE, however, is the switch to a self-sustaining situation, which is time-dependent. Seizures lasting more than 30 minutes would rarely stop spontaneously compared to 47% of those lasting between 10 to 29 minutes, which are self-resolving [50]. Moreover, in one study no self-limited seizure lasted more than 11 minutes [8].
The self-limiting character of seizures is due to inhibitory circuitry that suppresses their duration and propagation in the brain. Under specific circumstances, however, the inhibitory mechanisms fail and seizures progress to SE, which leads to synaptic reorganization, blood-brain barrier disruption, inflammation, metabolic crisis, more tissue damage, and further seizures. Neuronal injury during SE is the result of increased excitotoxicity [51–53] but also stems from systemic derangements such as hypoxia, acidosis, hypotension, or multiorgan dysfunction [54]. The seminal animal studies by Meldrum have shed a light on the systemic effects: after prolonged bicuculine-induced convulsive SE in baboons, neuronal damage and cell loss was evident in the neocortex, cerebellum and hippocampus. When systemic factors were kept within normal physiological limits (paralyzed and artificially ventilated animals with adequate serum glucose levels), there was decreased but still present neocortical and hippocampal cell damage, but absent cerebellar cell injury [55,56]. These experiments showed more than 40 years ago that the seizure activity per se is responsible for the neuronal damage and the systemic derangements play an additional role.
The direct neuronal injury as a result of the ongoing seizures, the perpetuation of seizures into SE, the resistance to treatment and the refractoriness that ensues have also been elucidated at a molecular level during the last decades. Initially, the g-aminobutyric acid (GABA) inhibitory circuits may be deficient and this is the reason why benzodiazepines or barbiturates, which work through GABAergic receptor agonism, are very effective during this early period. As time passes however, GABA receptors undergo a significant shift in their ability to respond to benzodiazepines [57,58]. This is due to changes in receptor presence at the inhibitory synapse, a phenomenon that has been called “receptor trafficking” by Arancibia and Kittler in 2009 [59]. There are differences in the type of GABAA receptors found synaptically and extrasynaptically. GABAA receptors containing the γ subunit are located synaptically and mediate phasic inhibition. Conversely, the δ subunit-containing GABAA receptors are located exclusively extrasynaptically and mediate tonic inhibition [60,61]. Smith and Kittler described the highly dynamic state of receptor presence on the surface of axons and explained how receptors move laterally from extrasynaptic sites to the synapse and then out of it to be internalized and either recycled to the surface or degraded [62]. This “receptor trafficking” intensifies during SE, and the overall effect becomes a reduction in the number of functional GABAA receptors in the synapses. As GABA is the principle inhib-itory transmitter, this reduction in GABAergic activity may be an important reason for seizures to become persistent.
However, this is not all. Additional mechanisms leading to refractoriness include the following:
(a) Excessive relocation of N-methyl-D-aspartate (NMDA)type glutamate receptors to the cell surface after 1 hour of SE, leading to increase of miniature excitatory NMDA currents and NMDA neurotransmission, with potentiation of glutamate excitotoxicity [53,63]
(b) Increased brain expression of drug efflux transporters, such as P-glycoprotein at the blood-brain barrier, which may reduce concentrations of AEDs at their brain targets [64]
(c) Up- and down-regulation of specific ATP-gated ion channels (P2X receptors) inducing altered response to ATP release [65]
(d) Change in the extracellular ionic environment (for example, the normally inhibitory GABAA receptor-mediated currents may become excitatory with changes in extracellular chloride concentrations) [66]
(e) Mitochondrial insufficiency or failure, which would lead to cell necrosis and apoptosis [67]
(f) Inflammatory processes, with opening of the blood-brain barrier (BBB) contributing to perpetuation of seizures [44]. The underlying mechanism is a maladaptive response of the astrocytes to the BBB damage, leading to activation of the innate immune system and disturbed homeostasis of the extracellular potassium and glutamate [68].
(g) Large-scale changes in gene expression within the affected brain regions; these are regulated by micro-RNAs, influencing protein levels playing a role in excitability, neuronal death and neuroinflammation [69].
All of these pathophysiologic derangements may become targets for future antiepileptic treatments.
Although the direct and indirect injury from ongoing convulsive SE is not in doubt, the significance of NCSE or the ictal-interictal continuum on inflicting additional injury has been more controversial. Recent data, however, do not support a benign process in these situations. It has been shown lately that nonconvulsive seizures lead to physiologic changes in the brain, including elevated intracranial pressure, changes in the brain metabolism, and delayed increase in cerebral blood flow [25]. In addition, using microdialysis, elevated lactate/puruvate ratio, indicating metabolic crisis, has been shown during periods of nonconvulsive seizures or periodic discharges [70]. Similarly, high-frequency periodic discharges lead to inadequate increase in cerebral blood flow and tissue hypoxia [71], and lateralized periodic discharges, lateralized rhythmic delta activity, and generalized periodic discharges are associated with seizures [72].
Diagnosis of SE
The diagnosis of SE is primarily clinical and encompasses motor phenomena and alteration of mental status. Focal-onset convulsions can remain focal, follow a Jacksonian march, or immediately generalize to involve the whole body with loss of consciousness. Most of the time, this secondary generalization can only be appreciated during EEG recording. In addition, mental status alteration can differentiate simple partial SE (no change in mental status) from complex partial SE (disturbed sensorium).
The presence or absence of motor phenomena and loss of consciousness do not necessarily correlate with the EEG activity during or after SE. For example, persistent electrographic seizures or NCSE after control of convulsive SE have been demonstrated with continuous EEG [73]. Conversely, altered mental status is also a poor clinical differentiator, since 87% of patients successfully treated for convulsive SE and 100% treated for NCSE remained comatose 12 hours following the initiation of therapy [20]. In addition, only 27% of motor, seizure-like phenomena in the ICU were proven to be seizures in a retrospective study [74]. Psychogenic nonepileptic attacks, occurring in between 3.8% and 9.5% of ICU patients presenting with seizures [74,75], is another situation that may lead to confusion, inappropriate intubation, and ICU admission. Strange phenomena, such as fasciobrachial seizures (brief facial grimacing and ipsilateral arm posturing) many times preceding the onset of amnesia, confusion, or temporal lobe seizures have been described in patients who have non-paraneoplastic limbic encephalitis associated with voltage-gated potassium channel (VGKC) antibodies, especially against the leucine-rich glioma inactivated-1 (LGI1) protein [76,77].Without a continuous video EEG, these phenomena may not be captured or appreciated. Therefore, EEG monitoring is an important tool for the evaluation of these patients and criteria for its use have been published [78]. The EEG criteria for convulsive SE have been clearly delineated, but for NCSE a mix of clinical and EEG criteria should be met [14,15,79].
In addition to clinical observation and EEG, there has been interest lately in multimodality monitoring of acutely brain-injured patients for seizures or SE using electrocorticography or mini depth electrode placement, partial brain tissue oxygen tension, cerebral blood flow, and microdialysis in addition to scalp EEG. Although preliminary and limited in few academic centers, this approach has produced interesting findings. For example, in a study from Columbia University, 38% of 48 patients with subarachnoid hemorrhage and multimodality monitoring had intracortical seizures, while only 8% of them had surface seizures, all nonconvulsive [25]. In another study, 68% of seizures and 23% of periodic discharges were only captured on the depth electrodes and were missed on the surface ones [71]. Therefore, detection of SE may change in the future with use of more sensitive techniques than scalp EEG.
Treatment
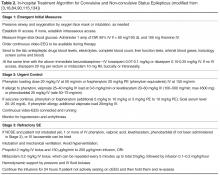
Significant practice variations exist in the management of SE even among academic centers in the US [80] despite the fact that the goals of treatment are concrete. These include (1) emergent medical management, (2) termination of seizures, (3) prevention of recurrence of seizures, and (4) prevention or treatment of complications.
Management of SE must begin in a prehospital setting by the emergency medical services, because the faster the treatment is offered, the better the response. Several studies have attempted to assess the possibility of aborting SE even prior to the hospital. In a randomized, double-blinded study, lorazepam was 4.8 times and diazepam 2.3 times more effective than placebo in terminating SE on arrival in the ED when given intravenously (IV) by paramedics [81]. The RAMPART study was a double-blind, randomized, non-inferiority trial comparing the efficacy of intramuscular (IM) midazolam (10 mg followed by placebo IV) with that of IM placebo followed by intravenous lorazepam (4 mg) for children and adults in SE treated by paramedics. At the time of arrival in the ED, seizures had ceased without rescue therapy in 73.4% and 63.4%, respectively, favoring midazolam [82].
Emergent Initial Phase
During the emergent initial phase, the goals are protection of the airway, oxygenation, maintenance of blood pressure, exclusion of easily
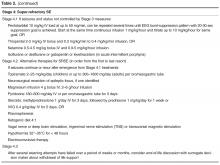
Urgent Control
If seizures continue, stage 2 medications should be used for benzodiazepine-refractory SE as urgent control treatment. There are some data suggesting better response rate to valproate after failure to control seizures with phenytoin than to phenytoin after failure of valproate [88]. If available, IV fosphenytoin is preferable to IV phenytoin due to potentially lower risk of side effects. Levetiracetam and phenobarbital IV are also acceptable choices. Levetiracetam can be administered as an off-label loading dose of 20–60 mg/kg IV (although the initial manufacturer was not supporting a “loading” dose; dose of up to 60 mg/kg IV up to 4500 mg maximum has been supported by the latest American Epilepsy Society guidelines [4]). This AED at an initial dose of 2–3 g/day confers an estimated success rate around 70% [89]. In a systematic review of 27 studies (798 cases of convulsive SE) comparing 5 AEDs in the treatment of benzodiazepine-resistant convulsive SE, phenobarbital and valproate had the highest efficacy (73.6% and 75.7%, respectively), followed by levetiracetam (68.5%) and phenytoin (50.2%). Lacosamide studies were excluded from the meta-analysis due to insufficient data [90], but its efficacy has been reported for patients with convulsive and NCSE [91,92]. There is not enough evidence at this point, however, to recommend its routine use for benzodiazepine refractory SE [90].
Refractory SE
When seizures continue despite the use of benzodiazepines and 2nd stage AEDs, SE becomes refractory (stage 3). Treatment of these resistant cases is frequently initiated in the ED and continued in an ICU. Outcomes were not significantly better in patients with SE admitted and managed in a neuro-ICU compared to a general medical ICU in a retrospective study, but the numbers were small (only 27% of SE were admitted to the former) [93] and this may change in the future. Intubation and mechanical ventilation is the first step, if not already present (only 21% of patients in the RAMPART study received endotracheal intubation, with 6.4% in the prehospital setting and 93.6% after admission [87]). Hemodynamic support with pressors or inotropes may be required as most anesthetic agents may decrease the blood pressure. Because of the urgency of controlling the seizures during SE, the potential aspiration risk and the questionable enteral absorption per os administration of additional AEDs is problematic, and IV formulations should be used.