Geriatric Oncology Comes of Age
It’s not just a question of tolerating classic chemotoxicities, however. "If they’re already having trouble getting around their apartment and they fell once a year ago ... any kind of assault on the body may lead to a decompensation," said Dr. Goodwin.
The CARG Chemotoxicity Risk Score
Dr. Hurria and her coinvestigators followed 500 cancer patients 65 years and older throughout chemotherapy to find factors predictive of chemotoxicity, and used their findings to develop the Cancer and Aging Research Group (CARG) Chemotoxicity Risk Score.
Age, cancer type, chemotherapy dose, number of chemotherapy drugs, anemia, and low creatinine clearance were among 11 key factors identified by the study. The rest were based on the answers to five key questions assessing various geriatric measures: self-rated hearing, number of falls in the last 6 months, need for help in taking medications, ability to walk one block, and level of social activities.
Dr. Hurria reported a significant association between the patients’ baseline risk scores and their subsequent risk of grade 3-5 chemotherapy toxicity. Those with a score greater than 12 had an 83% overall risk of grade 3-5 toxicity,compared with 27% for those with a score of 0-5. The investigators divided the study population into low-risk, intermediate-risk, and high-risk categories based on the scores.
Cancer patients 65 years and older were eligible for the study, if they were going to start a new chemotherapy regimen. Geriatric assessment began prior to the start of treatment.
Mean patient age was 73 years, 85% were white, and 56% were women. Nearly two-thirds had stage IV disease, and 70% were on polychemotherapy. In all, 53% had grade 3-5 toxicities due to chemotherapy – of these, 26% had hematologic and 43% had nonhematologic toxicities. The majority of toxicities were grade 3
The study captured laboratory values, documented the chemotherapy the patient underwent – the number of drugs, normal or modified dosing, first-line therapy or other – and asked a host of questions in a geriatric assessment that included:
• Functional status (Activities of Daily Living, Instrumental Activities of Daily Living [IADL], Karnofsky Performance Rating Scale, timed up-and-go test [time needed to rise from a chair and start walking], the number of falls in the last 6 months).
• Comorbidity.
• Cognition (Blessed Orientation-Memory-Concentration Test).
• Psychological state (Hospital Anxiety and Depression Scale).
• Social functioning (Medical Outcomes Study Social Activity Limitations Measure).
• Social support (Medical Outcomes Study Social Support Survey, Seeman and Berkman Social Ties).
• Nutritional status (body mass index, percentage unintentional weight loss in the last 6 months).
"We really looked at what are the factors – other than chronologic age – that can tell us about this individual," said Dr. Hurria.
Two physicians graded chemotherapy toxicities using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v3.0) to determine whether the event was due to disease or chemotherapy. Patients were followed until the end of the regimen.
"We felt that in order for a measure to be utilized in clinical practice, we would need to increase both the ease of administration and the ease of the scoring by having health care providers be able to ask single questions to the patient in order to assess their risk," she said.
Therefore, the researchers developed the scoring algorithm, assigning points to each risk factor, and then used the whole cohort to determine how total score relates to chemotoxicity risk.
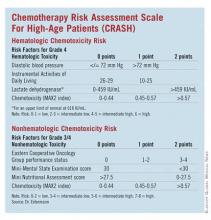
The CRASH Score
Developed by Dr. Extermann and her coinvestigators, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score also evaluates an elderly patient’s risk for grade 4 hematologic and grade 3/4 nonhematologic toxicities during chemotherapy.
"This score is helping define – a priori – the level of risk that a patient has for side effects. This way, we know how closely we need to watch the patient during chemotherapy, what strength of chemotherapy we should give, or if we should give chemotherapy at all," said Dr. Extermann.
Investigators enrolled patients from the Moffitt Cancer Center and six community centers. The process included a baseline assessment within 2 weeks of starting chemotherapy and weekly complete blood cell counts. Any published chemotherapy regimen could be used, and clinicians were free to manage care without restriction.