Development, implementation, and evaluation of a prostate cancer supportive care program
Many men who are diagnosed with prostate cancer face long-term treatment-related health effects that will affect their quality of life and have cost implications for the health system. In this article, we describe and assess the use of and satisfaction with the Prostate Cancer Supportive Care (PCSC) Program, which is a comprehensive, evidence-based, modular program that aims to address these concerns. We include data from patient medical records, PCSC Program registration forms and attendance records, and anonymized participant feedback forms. We examine the clinical and sociodemographic characteristics of program participants, program participation rates, and satisfaction with individual program modules. Among the 1,269 registrants, 1,206 (84%) participated in the program. Modules that provided information on prostate cancer and treatment options and offered sexual health support had the most participants (29% and 55% of total program participants, respectively). Satisfaction with all program components was high among both survivors and their partners (average score, 3.6 out of 4). Robust evaluations of the program's effects on quality of life and health system costs are ongoing. There is a growing need to provide consistent and comprehensive support to prostate cancer survivors and their partners and families. As such, we recommend that alongside direct oncologic care, clinicians assess their patients' needs for supportive care services and refer them to programs that will provide comprehensive support throughout the disease and treatment journey. Funding The Michael Smith Foundation for Health Research (grant number 16605) and Prostate Cancer Canada (grant number PDF2016-1270)
Accepted for publication November 20, 2018
Correspondence Lindsay Hedden, PhD; lindsay.hedden@ubc.ca
Disclosures Dr Elliot served on the board for Aceras, and has given a lecture for Pentopharm. Dr Higano has received research grants from Aptero, Aragon, Astellas, Astra-Zenica, Bayer, Dentreon, Hoffman-Laroche, Medivation, and Pfizer. Dr Higano also served on the advisory board or in a consultancy role for Astellas, Bayer, Blue Earth, Cloris, Orion, and Tolmar.
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0438
Program participation
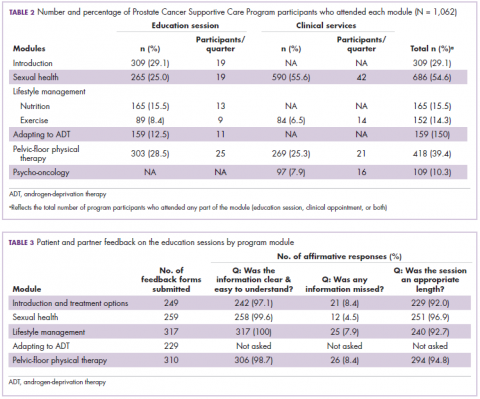
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
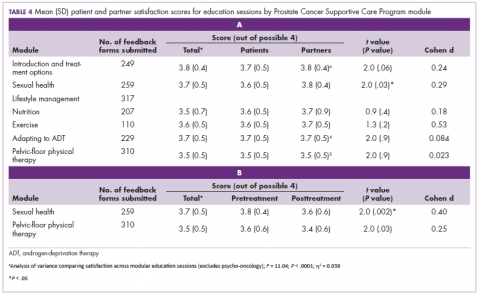
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
,Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.