APF530 for nausea and vomiting prevention following cisplatin: phase 3 MAGIC trial analysis
Background APF530 is approved for preventing acute and delayed chemotherapy-induced nausea and vomiting (CINV) associated with initial and repeat moderately emetogenic chemotherapy or anthracycline plus cyclophosphamide (AC) regimens, based on phase 3 trials.
Objective To evaluate APF530 for CINV among cisplatin-stratum patients in the phase 3 MAGIC trial.
Methods Stratification was by planned receipt of cisplatin, with randomization to APF530 500 mg SC or ondansetron 0.15 mg/kg IV. Patients received fosaprepitant 150 mg IV plus dexamethasone 12 mg IV (day 1) and oral dexamethasone 8 mg (once, day 2; twice daily, days 3-4). The primary endpoint was delayed-phase complete response (CR). Other endpoints included CR, complete control (CC), and total response (TR) across phases, time to first rescue medication use, proportion of patients with no rescue medication use, and nausea frequency. Adverse event (AE) assessments included injection-site reactions (ISRs). This analysis evaluated cisplatin-stratum patients.
Results 264 of 942 randomized patients were included in the cisplatin stratum, 252 in the efficacy analyses (124 APF530, 128 ondansetron). Delayed-phase CR was numerically higher with APF530 than ondansetron, with a 10.6% treatment difference (APF530: 65.3% [81/124]; ondansetron: 54.7% [70/128]; P = .085). Similar trends favored APF530 for CC, TR, rescue medication use, and nausea endpoints. APF530 was well tolerated; most AEs were ISRs, generally mild or moderate.
Limitations Exploratory analysis, not powered to detect significant between-arm differences.
Conclusions Consistent with significant results in the overall population, APF530 showed clinical benefits in CINV control in patients scheduled for cisplatin-based regimens.
Funding Heron Therapeutics Inc, maker of the study drug, APF530.
Accepted for publication February 13, 2017
Correspondence Lee Schwartzberg, MD, FACP; lschwartzberg@westclinic. com
Disclosures Dr Schwartzberg has received consulting fees from Heron Therapeutics, the maker of the study drug, outside of the submitted work. Dr Mosier has received personal fees for statistical analysis from EMB Statistical Solutions LLC, outside the submitted work. Dr Geller received personal fees from Heron Therapeutics, during the conduct of the study. Dr Klepper received consulting fees from Heron Therapeutics during the conduct of the study, and consulting fees from Heron Therapeutics, outside the submitted work. Dr Schnadig is the scientific advisor and consultant to Medical Affairs Team at Heron Therapeutics. Dr Vogelzang is a consultant to Heron Therapeutics.
Citation JCSO 2017;15(2):82-88
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0331
Related articles
Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy
Prescriber adherence to antiemetic guidelines with the new agent trifluridine-tipiracil
Submit a paper here
Eligible men and women were 18-80 years of age with histologically or cytologically confirmed malignancy (cancer type information was not captured) and were entering the first cycle of their single-day HEC treatment (defined by ASCO 2011 emetogenicity criteria).7 Patients had Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0 or 1, no history or presence of significant cardiac disease or QT interval prolongation, and adequate bone marrow, kidney, and liver function. All patients provided written informed consent.
Procedures
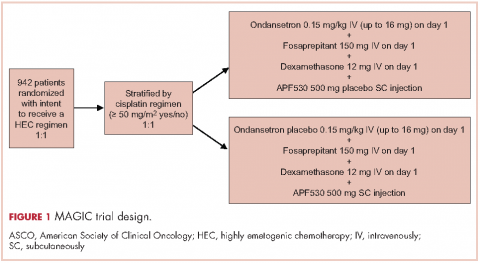
Patients were stratified by planned receipt of the cisplatin regimen ≥50 mg/m2 (Yes/No), randomized 1:1 to receive APF530 500 mg SC (granisetron 10 mg) or ondansetron 0.15 mg/kg IV (up to a maximum of 16 mg as a single dose) on day 1 (Figure 1). The APF530 arm received the ondansetron saline placebo, and the ondansetron arm received the APF530 SC placebo containing the TEG-POE vehicle. All patients were scheduled to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then oral dexamethasone 8 mg once daily on day 2 and 8 mg twice daily on days 3 and 4. Rescue medication was allowed at the investigator’s discretion.
Outcomes
The primary objective of the trial was to demonstrate the superiority of APF530 500 mg SC compared with ondansetron 0.15 mg/kg IV, as part of the current guideline-recommended 3-drug regimen, in preventing delayed-phase CINV after HEC. The primary endpoint was delayed-phase (24-120 h) CR (no emetic episodes [vomit or retch] and no rescue medication use). In addition, a prespecified analysis of delayed-phase CR by randomization strata (planned use of cisplatin) was performed.
,Secondary and other endpoints included overall-phase CR
(0-120 h); delayed-, overall-, and acute-phase complete control (CC: CR and no more than mild nausea); delayed-, overall-, and acute-phase total response (TR; CR and no nausea); and rescue medication use. A post hoc analysis of nausea severity was also conducted. Safety assessments included treatment-emergent adverse events (TEAEs), injection-site reactions (ISRs), laboratory parameters, and vital signs. TEAEs were assessed by type, duration, severity, and relationship to study drug. ISR timing and severity were captured in patient diaries.
Statistical analysis
All efficacy analyses were conducted using the modified intent-to-treat population (mITT; randomized patients who received study drug and a HEC regimen and had post-baseline efficacy data). Safety assessments were performed on the safety population (randomized patients who received study drug).
This analysis conducted on the subgroup of patients with intent to receive cisplatin (cisplatin randomization stratum, ≥50 mg/m2, Yes) was exploratory and was not powered to detect treatment differences. Preplanned analyses compared CR, CC, and TR rates across treatment arms using 95% CIs.
Post hoc analyses of time to first rescue medication use, proportion of patients with rescue medication use, and less frequent nausea were performed. All P values were calculated using the Cochran-Mantel-Haenszel chi square test. Rescue medication use results were based on observed data, without imputation for missing results (ie, calculated from the number of patients with a response). Further analyses of efficacy endpoints CR, CC, and TR in the subset of female patients in the cisplatin randomization stratum were performed. Safety assessments were summarized descriptively.
Results
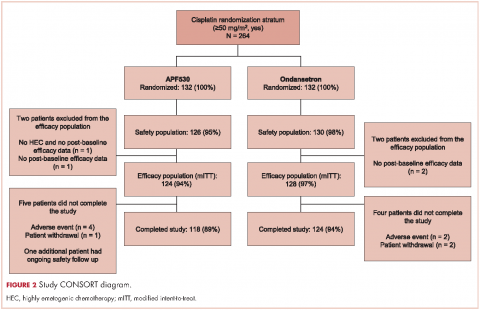
A total of 942 patients were randomized across 77 US centers during March 31, 2014 and May 15, 2015 (471 APF530, 471 ondansetron). Among those, 264 had intent to receive cisplatin and were included in the cisplatin randomization stratum (≥50 mg/m2, Yes) (Figure 2). A total of 256 patients in the cisplatin stratum received study drug and were included in the safety population (126 APF530, 130 ondansetron); 252 patients were included in the mITT population (124 APF530, 128 ondansetron).
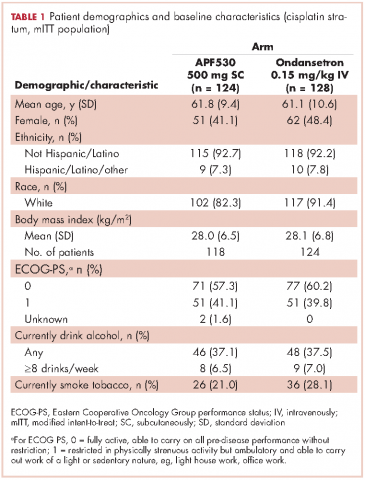
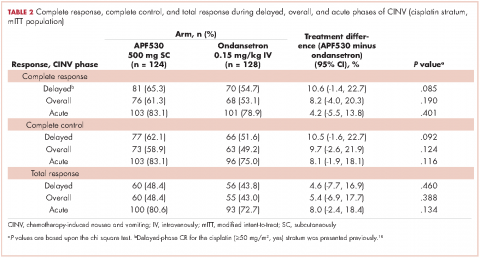
Baseline demographics were generally balanced between treatment arms (Table 1). The proportion of female patients was 41.1% (51/124) and 48.4% (62/128) in the APF530 and ondansetron arms, respectively. The majority of patients had an ECOG PS of 0 (57.3% [71/124] APF530; 60.2% [77/128] ondansetron). The most common cisplatin-based chemotherapy regimen in both treatment arms was cisplatin and gemcitabine (25.0% [31/124] APF530; 28.9% [37/128] ondansetron) (Suppl Table 1). Two patients in the APF530 arm and 3 patients in the ondansetron arm either received a lower cisplatin dose (<50 mg/m2) or did not go on to receive cisplatin as intended at randomization (Suppl Table 1). As previously reported, in the cisplatin stratum (Table 2), delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, with a corresponding treatment difference of 10.6% (65.3% [81/124] APF530; 54.7% [70/128] ondansetron; 95% CI [-1.4, 22.7]; P = .085). Although the CI contains 0, the result is consistent with the significant benefit observed in the overall study population (64.7% [291/450] APF530; 56.6% [256/452] ondansetron; 95% CI [1.7, 14.4];
P = .014).18 This more in-depth analysis found similar trends favoring the APF530 over the ondansetron regimen across overall- and acute-phase CR (Table 2).
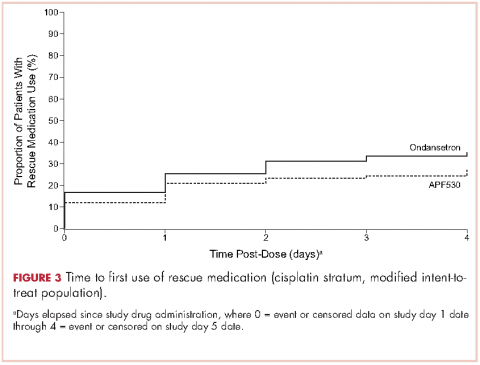
A significantly greater proportion of patients in the APF530 arm, compared with the ondansetron arm, reported no rescue medication use during the delayed phase (74.4% [90/121] APF530; 62.6% [77/123] ondansetron; P = .048). Trends in favor of APF530 were observed in the overall phase (71.1% [86/121] APF530; 61.8% [76/123] ondansetron; P = .125) and acute phase (86.9% [106/122] APF530; 81.9% [104/127] ondansetron; P = .278). Time to first rescue medication use was consistently longer in the APF530 arm, compared with the ondansetron arm, although not statistically significantly (P = .150) (Figure 3).
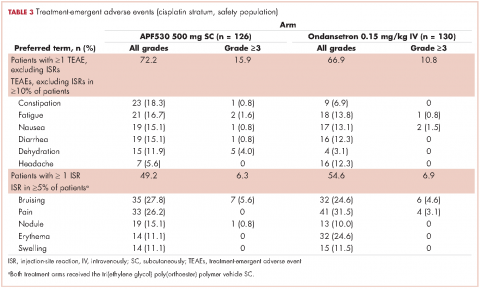
The APF530 regimen was generally well tolerated in the cisplatin subgroup, and no new safety signals were identified (Table 3). Most patients experienced at least one TEAE. Excluding ISRs, TEAE incidences were 72.2% and 66.9% in the APF530 and ondansetron arms, respectively; most common were constipation, fatigue, nausea, diarrhea, dehydration, and headache. Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (2.4% and 2.3%, respectively and headache (3.2% and 4.6%).