Single-, low-dose cyclophosphamide-associated severe hyponatremia with seizures in a patient with breast cancer
Accepted for publication November 22, 2016
Correspondence Shou-Ching Tang, MD, PhD; stang@augusta.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(1):37-39
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0319
Related articles
Social support needs among patients with advanced breast cancer: sensitivity trumps substance
Lessons learned from using CDK 4/6 inhibitors to treat metastatic breast cancer
Submit a paper here
Discussion
Hyponatremia is a common finding in cancer patients caused usually by paraneoplastic syndrome, chemotherapy, immunotherapy, or other associated treatment.6 SIADH is a frequent cause of hyponatremia in cancer patients and should be suspected in patients with hyponatremia, hypo-osmolality, and a urine osmolality above 100 mOsmol/kg.7
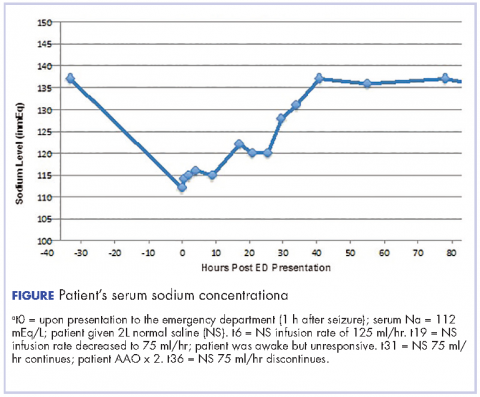
Our patient’s presentation and laboratory findings suggested SIADH as the likely cause of hyponatremia with a low sodium, a serum osmolality 242 mOsm/kg and urine osmolality of 449 mOsm/kg.8-10 She had no known underlying contributory comorbid condition relating to her serum lipids, thyroid, adrenal, kidney, or heart to date. Her use of a thiazide diuretic was the only confounding factor. The most plausible cause of hyponatremia/SIADH in our patient was likely cyclophosphamide based on her history, timeline of symptoms, and the absence of other possible causes. Though the mechanism for many of the previously mentioned etiologies are known, the mechanism of cyclophosphamide-induced SIADH is difficult to elucidate since the imminent complication of hemorrhagic cystitis means patients receiving this drug are often aggressively hydrated to prevent this complication.11,12 The result is that there is marked retention of water leading to potentially fatal hyponatremia in selected cases.11 This phenomenon has been fairly well described in patients receiving doses of 6 g/m2 as given in the STAMP protocol for stem cell mobilization or at doses of 30-50 mg/kg used to treat malignancy.12 Our patient clearly falls in this category given that she received a dose of 600 mg/m2. We found no evidence in her history to suggest post-operative, genetic or other cause for her hyponatremia. Our case mirrors a report by Koo and colleagues who described severe hyponatremia occurring within 24 hours following a single dose of intravenous cyclophosphamide 700 mg followed by saline infusion.13 In the case reported by Jayachandra and colleagues in which suspected cyclophosphamide-induced hyponatremia led to seizures, the patient received 500 mg IV of cyclophosphamide and had serum sodium as low as 106 mEq/L within a 24-hour period,2 similar to our patient.
There is a paucity of data on cyclophosphamide-induced SIADH. The mechanism by which cyclophosphamide causes SIADH is currently unknown. In addition, there are currently no set criteria that help identify at-risk patients who may develop such an event, including the dosage of cyclophosphamide that may trigger the SIADH, because lower doses of the drug have been associated with this complication.14
In a retrospective analysis by Lee and colleagues, cyclophosphamide-induced hyponatremia was found to be associated with male sex on a univariate analysis, but no risk factors were found in a multivariate analysis.15 It is likely that the concomitant use of diuretics, hydration, and high-dose cyclophosphamide contributed to hyponatremia/SIADH in our patient, though it is not clear through what mechanism. Harlow and colleagues proposed a mechanism for this phenomenon in 1979 based on the autopsy of a patient who had received high-dose cyclophosphamide involving degranulation of hypothalamic neurosecretory organelles and loss of Herring’s bodies. They inferred that metabolites of cyclophosphamide indirectly triggered inappropriate secretion of antidiuretic hormone as seen with a use of the structurally related analogue ifosfamide,16 but to our knowledge, this has yet to be replicated. Cyclophosphamide metabolite may have a direct tubular effect on the collecting duct epithelium leading to water retention15 as established by Campbell and colleagues. In one case, an established diabetes insipidus patient developed cyclophosphamide-induced antidiuresis without vasopressin secretion.17 It is imperative that the scientific community conduct research into the risk factors, underlying mechanisms, and methods of prevention to reduce and/or eliminate SIADH associated with use of cyclophosphamide.