Paraneoplastic leukemoid reaction – poor prognostic marker in urothelial bladder carcinoma
Accepted for publication March 14, 2016
Correspondence Mark Ashamalla, MD; Mark.Ashamalla1@gmail.com
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(1):40-42
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0264
Submit a paper here
Certain cancers have been observed to cause symptoms called paraneoplastic syndromes that are not directly attributed to tumor invasion or compression. This phenomenon is believed to be secondary to a tumor’s secretion of functional hormones, peptides, cytokines, or its immune cross-reactivity. One such variant is a paraneoplastic leukemoid reaction (PLR), defined as a leukocytosis level of >50 x 103 cells/mm³, where the white blood cell (WBC) count differential exhibits a neutrophilia or left-shift, in which a predominance of early neutrophil precursors is observed. A PLR is believed to be incited by the tumor cell’s production of its own growth factors such as granulocyte-colony stimulating factor (G-CSF) and a number of different cytokines. These reactions may at first be mistaken for infectious processes, and it is only after an infection has been ruled out or when a leukocytosis is disproportionately high in the setting of a treated infection, that a paraneoplastic leukemoid reaction (PLR) is considered and an oncologic work-up pursued.
PLR has been previously described in a variety of malignancies including lung, esophageal, nasopharyngeal and laryngeal, gastric, cholangiocarcinoma, melanoma, multiple myeloma, renal, prostate, and hepatocellular carcinoma, but has rarely been described in urothelial carcinoma.1 Leukemoid reactions and autocrine growth induced by paraneoplastic production of G-CSF have rarely been associated with urothelial carcinoma of the bladder,2 as in the case we present here of a 67-year-old white man with invasive high-grade urothelial carcinoma of the bladder. The case highlights PLR as a negative prognostic marker, secondary to urothelial bladder cancer cells’ presumed production of G-CSF, rarely reported as in the literature previously.
Case presentation and summary
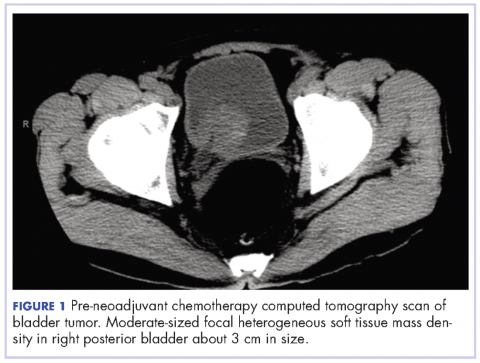
A 67-year-old white man was diagnosed with clinical stage III (T3N0M0), invasive high-grade urothelial carcinoma of the bladder (Figure 1). He received neoadjuvant chemotherapy with the standard gemcitabine-cisplatin combination (1,000 mg/m2 of gemcitabine on days 1, 8, and 15 with 70 mg/m2 of cisplatin on day 1 of a 28-day cycle for 3 cycles), and had less than partial response at the end of a 3-month course. A computed tomography scan of his pelvis obtained at treatment completion revealed persistent disease with noted enhancement of the right distal ureter and a right posterior bladder mass at the ureterovesical junction measuring 1.7 x 2.6 x 2.6 cm (0.6 x 1 x 1 in), for which, a cystoprostatectomy was recommended to remove remaining disease. The patient was seen in routine follow-up 3 weeks after his last chemotherapy treatment, when his WBC count was noted to be within normal limits at a value of 8.4 x 103 cells/mm³ (normal range, 4.5-11 x 103 cells/mm³).
,One week later (a month after treatment completion), the patient presented to the emergency department with complaints of dysuria, urinary frequency, and suprapubic pain. He was found to have leukocytosis, with a WBC count of 47 x 103 cells/mm³, (normal range, 4.5-11 x 103 cells/mm³), with an elevated neutrophil count of 82.7% (normal range 40%-60%), without clinical signs of systemic infection (fevers, chills, or rigors). A urinalysis revealed pyuria with 25-50 WBC/high power field, negative nitrite, positive leukocyte esterase, and moderate bacteria, consistent with what was presumed to be a urinary tract infection. The patient was discharged home with a 1-week course of the antibiotic levofloxacin and the alpha-blocker tamsulosin to make urination easier. Of note, the final results of the urine culture, which returned 48 hours after discharge, showed no growth.
One week prior to surgery, the patient underwent a cystoscopy for ureteral stent exchange, which revealed a necrotic tumor at the right bladder base surrounding the ureteral orifice and stent. Renal pelvis urine, sampled during stent exchange, revealed >100,000 CFU/ml (colony forming units; normal value, <10³) Candida albicans, for which the patient was started on intravenous fluconazole for fungal infection. We consulted with our colleagues in the infectious disease department and continued to follow the patient throughout his hospital course, which included several antibiotic regimens, a comprehensive hematological work-up and eventual urologic surgery. Work-ups for myeloproliferative disorders, leukemia, JAK-2, and BCR-ABL were all negative. A peripheral blood smear analysis showed mostly neutrophils, no immature cells, and occasional hypersegmented neutrophils, but was overall inconsistent with myeloproliferative disease. Despite the patient’s persistent leukocytosis, he remained completely asymptomatic and his neutrophilia was attributed to his malignancy.
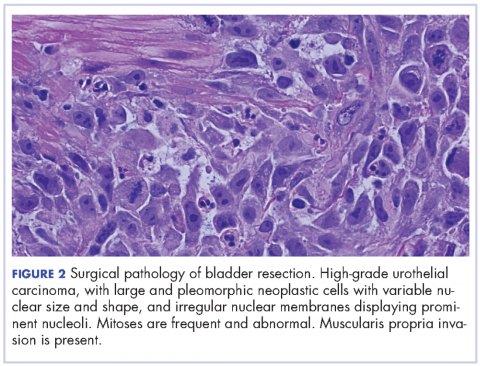
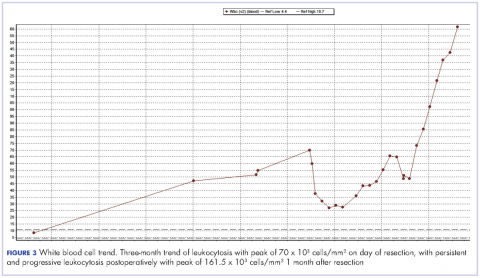
The patient subsequently underwent a cystoprostatectomy with ileal conduit. The surgical pathologic analysis showed a high-grade, invasive urothelial (transitional cell) carcinoma measuring 5.2 x 5.0 x 4.5 cm (2 x 1.9 x 1.8 in) with squamous differentiation, extensive tumor necrosis, lymphovascular invasion, invasion into the adjacent seminal vesicle and prostatic stroma, and negative margins (pT4a pN0 pMX; Figure 2). On the day of surgical resection, the patient’s leukocytosis was 70 x 103 cells/mm³.
Despite a transient improvement in his leukocytosis to 37.7 x 103 cells/mm³ on postoperative day 1, the patient remained in the medical intensive care unit for uncontrolled pain and management of his leukocytosis. It is worth noting that the patient remained afebrile throughout his entire 45-day hospitalization, with negative culture data, and despite receiving an extensive broad spectrum antibiotic regimen (levofloxacin, piperacillin-tazobactam, cefazolin, metronidazole, ceftriaxone and fluconazole), his leukocytosis continued to progress, peaking at 161.5 x 103 cells/mm³ less than a month after his surgery (Figure 3).
The patient continued to deteriorate rapidly, with a progressive leukocytosis, and developing metastases to the lung, perineum, and penis. He died a month after surgery (2 months after completion of neoadjuvant chemotherapy). The leukocytosis exhibited in this patient and the aggressive tumor cell growth are believed to have been secondary to a paraneoplastic leukemoid reaction incited by the urothelial bladder cancer cells’ presumed production of G-CSF, which has been rarely reported in the literature previously.