Therapeutic management of NAFLD
Management of comorbidities
Given the multiple comorbidities associated with NAFLD and the potential to influence its severity, a comprehensive and multidisciplinary approach is needed to ameliorate not only the progression of liver disease but also those complications related to metabolic syndrome, hyperlipidemia, hypertension, diabetes, and other related conditions. Of note, all patients with NAFLD should receive aggressive management of comorbidities regardless of the severity of NAFLD. Ideally, a multidisciplinary team – including a primary care provider, an endocrinologist for patients with T2D, and a gastroenterologist/hepatologist – is needed to successfully manage patients with NAFLD.
It is well recognized that individuals with biopsy-proven NAFLD are at a higher risk of coronary heart disease, stroke, congestive heart failure, and death resulting from CVD when compared with the non-NAFLD population, and excess in CVD morbidity and mortality is evident across all stages of NAFLD and increases with worsening disease severity.34 The strong association between CVD and NAFLD has important clinical implications that may influence the decision to initiate treatment for primary prevention, including lipid-lowering, antihypertensive, or antiplatelet therapies.35 Statins are widely used to reduce LDL cholesterol and have been proven to be safe in NAFLD, including for those with elevated liver enzymes and even in compensated cirrhosis, in several studies conducted during the last 15 years.36 Statins are characterized by anti-inflammatory, anti-oxidative, antifibrotic, and plaque-stabilizing effects, whereby they may improve vascular and hepatic function among patients with NAFLD and reduce cardiovascular risk.37 Statin use for the treatment of NAFLD is still controversial and off-label and is not specifically recommended to treat NASH, but positive results have been shown for reductions in liver enzymes.1 A recent meta-analysis of 13 studies showed that continued use of statin in cirrhosis was associated with a 46% and 44% risk reduction in hepatic decompensation and mortality, respectively.38
The Food and Drug Administration has approved omega-3 (n-3) fatty acid agents and fibrates for the treatment of very high triglycerides (500 mg/dL or higher); however, no specific indications exist to treat NAFLD.1 Fenofibrate is related to mild aminotransferase elevations and, in some cases, severe liver injury, so caution must be paid, especially within 2 days of taking the drug.39-40
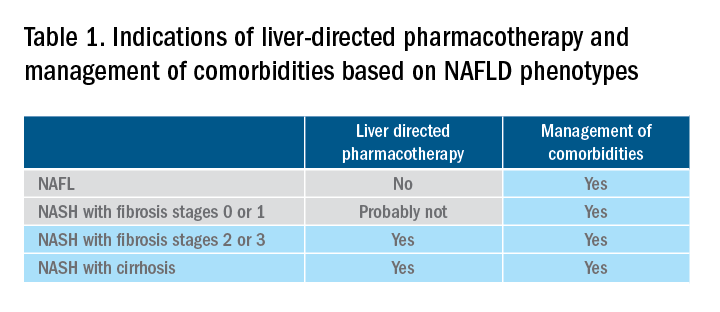
NAFLD phenotypes that need liver pharmacotherapy
There are still no FDA-approved drugs or biological treatments for NASH. Pharmacological interventions aiming primarily at improving liver disease should generally be limited to those with biopsy-proven NASH and clinically significant fibrosis (fibrosis stages of 2 or greater).1,4 For FDA approval, medications used for treating NAFLD with fibrosis need to meet one of the following endpoint criteria: resolution of NASH without worsening of fibrosis, improvement in fibrosis without worsening of NASH, or both. In addition to those criteria, a new medication might improve the metabolic profile and have a tolerable safety profile. Table 1 displays those NAFLD phenotypes that will likely benefit from liver-directed therapy.
Obeticholic acid as an experimental therapy for NASH
A planned month-18 interim analysis of a multicentre, phase III RCT examined the efficacy and safety of obeticholic acid (OCA), a farnesoid X receptor agonist, in patients with NASH and stages 1-3 of fibrosis. The primary endpoint (fibrosis reduction 1 stage or more with no worsening of NASH) was met by 12% of patients in the placebo group, 18% of patients receiving OCA 10 mg (P = .045), and 23% of those receiving OCA 25 mg (P = .0002). An alternative primary endpoint of NASH resolution with no worsening of fibrosis was not met. OCA 25 mg led to the highest rates of pruritus and hyperlipidemia, compared with OCA 10 mg.42 These side effects seem to be related to the activation of the farnesoid X receptor.43







