Guideline Concordance with Durvalumab in Unresectable Stage III Non-Small Cell Lung Cancer: A Single Center Veterans Hospital Experience
Background: Durvalumab is recommended by national guidelines for patients with unresectable stage III non-small cell lung cancer (NSCLC) following concurrent chemoradiation therapy (CRT). Nonadherence to guidelines is associated with adverse outcomes. We studied the adherence and identified barriers to durvalumab usage at the Birmingham Veterans Affairs Medical Center (VAMC) Oncology Clinic in Alabama.
Methods: Using retrospective analysis, we assessed the use of consolidative durvalumab among veterans at Birmingham VAMC. The health records of all veterans with stage III unresectable NSCLC from October 2017 to August 2019 were reviewed. Data collected included demographics, barriers to CRT initiation and completion, durvalumab usage, and reasons for not prescribing durvalumab.
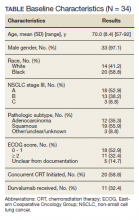
Results: In our data review, 34 patients were found to have stage III unresectable NSCLC. Twenty (58.8%) of those 34 initiated CRT, but only 16 (47.1%) completed CRT treatment and 7 (20.6%) underwent further treatment with durvalumab. Of the 14 patients who did not initiate CRT, the most common reasons were poor performance status and/or the presence of comorbidities. Of the evaluable cohort of 34, 11 (32.4%) patients with stage III NSCLC received durvalumab. Of the 9 eligible patients who did not receive durvalumab, the most common reasons cited were toxicities experienced during or following CRT (11.8%).
Conclusions: Just one-third of patients were eligible to receive durvalumab at Birmingham VAMC. This was likely due to the difference between clinical trial and real-world patient populations. Interventions to address socioeconomic and system level barriers to improve our center’s delivery of lung cancer treatment are planned.
Durvalumab Treatment
After initiation of CRT, 9 (26.5%) patients did not go on to receive durvalumab. Three patients (8.8%) suffered toxicities during CRT. One study patient was found to have a severe respiratory infection requiring intensive care unit admission. Another study patient was found to have a new sternal lesion on follow-up positron emission tomography. One declined because of a history of severe antineutrophil cytoplasmic antibodies vasculitis, which made durvalumab use unsafe. Three patients (8.8%) declined treatment with CRT or durvalumab because of personal preference. Documentation was unclear as to why durvalumab was prescribed to one patient who had completed CRT.
Discussion
NCCN guidelines on the use of durvalumab in NSCLC are based on the phase 3 PACIFIC placebo-controlled randomized clinical trial. This trial, which included only patients with documented performance status of ECOG 0 or 1, reported that grade 3 or 4 events occurred in 30.5% of patients randomized to consolidative durvalumab. Treatment was discontinued in 15.4% of patients due to adverse events.3
Our study examined consolidation therapy with durvalumab in patients with unresectable stage III NSCLC with an ECOG performance status of 0 to 1 who had not progressed after 2 or more cycles of definitive concurrent CRT.4 Patients with previous exposure to immunotherapy, a history of immunodeficiency, active infection, unresolved toxicity from CRT, autoimmune disease, and patients who received sequential CRT were excluded.2 Surprisingly, the adherence rate to guidelines was close to 100% with appropriate documentation and justification of CRT initiation and durvalumab use. Five (14.7%) of veterans with unresectable stage III NSCLC did not have clear documentation of ECOG status on initial visit and only 1 veteran who completed CRT did not have clear documentation as to why durvalumab was not provided. Unfortunately, 23 (68.6%) veterans in the study were unable to receive durvalumab, a potentially disease-modifying drug; nearly one-third (10) of veterans were deemed poor candidates for concurrent CRT despite the fact that 52.9% (18) of veterans in the study had a documented ECOG of 0 or 1 on initial evaluation.
Clinical Trials vs Real World
The heterogeneity between anticipated study populations, those who were able to receive durvalumab in the PACIFIC trial, compared with our observed real-world veteran population, likely stems from the lack of information about how comorbidity and fitness can affect the choice of therapeutic intervention in patients with lung cancer.12 In addition, older adults who participated in randomized controlled trials (RCTs) are not representative of the average older adult who presents to medical oncology clinics, making the application of guideline concordant care difficult.13
Similar real-world observations parallel to our analyses have confirmed, complemented and/or refuted findings of RCTs, and have helped impact the treatment of multiple acute and chronic conditions including influenza, cardiovascular disease, and diabetes.14
A component of socioeconomic barriers and access to supportive care played roles in the decisions of certain patients who chose not to undergo concurrent CRT despite medical advice. These 2 obstacles also affected the decision making for some in the study when considering the use of durvalumab (administered by a 60-minute IV infusion every 2 weeks for 1 year) per recommended guidelines.1 These hurdles need further study in the context of their effect on quality of life and the difficulties generated by various social determinants of health.