VA National Precision Oncology Program
Background: The US Department of Veterans Affairs (VA) is the largest integrated health care system in the US. The VA sees about 50,000 new cancer diagnoses each year and provides care for more than 400,000 veterans with cancer. The heterogeneity of molecular testing practice patterns and methods of testing at VA health care facilities and the increasing number and complexity of molecular tests led to the development of national program for precision oncology.
Observations: The National Precision Oncology Program (NPOP) provides tumor sequencing and consultative services for the treatment of veterans with cancer. NPOP is used by nearly all VA oncology practices and has now sequenced > 13,000 samples. The initial focus was on advanced-stage nonsquamous non-small cell lung cancer, which has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, testing was expanded to include metastatic prostate cancer and hematologic malignancies with prior approval. The program also offers cell-free DNA (liquid biopsy) and PD-L1 immunohistochemistry analyses.
Conclusions: The VA NPOP is one of the largest clinical DNA sequencing programs in the US with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cutting-edge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
Informatics
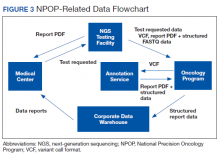
Informatics is an essential component of NPOP that facilitates both clinical care and research (Figure 3). Results of NGS gene panels are returned to the facility that submitted the sample for testing as a PDF document. NPOP receives the same PDF report in real time but also structured data of the results including a variant callformat file and XML file. The secondary sequence data in binary alignment map or FASTQ format is received in batches. NPOP program staff extract data from these files and then load it into SQL tables in the VA Corporate Data Warehouse. In partnership with the VA Pharmacy Benefits Management Service, NPOP has constructed user-friendly dashboards that allow users with no technical skills and who have the appropriate data access permissions to view various portions of the NPOP database. There are dashboards to display a list of NPOP samples by facility, find a patient by name or other identifying information, and display a list of patients who have received any antineoplastic drug, among other functions.
The NPOP database has been used to reannotate NGS results, such as when new drugs are approved. For example, when the first neurotrophic tropomyosin receptor kinase (NTRK) inhibitor was approved, NPOP rapidly identified all living patients with NTRK fusions and notified the health care providers of the availability a potential new treatment option for their patient. 7 NPOP is now building a method to allow NPOP dashboard users to similarly identify patients who have not received a corresponding drug for a user-selected LoE annotation. This will facilitate a systems approach to ensure that all patients with EGFR exon 19 deletions, for example, either have received an EGFR inhibitor or are reviewed to determine why they have not. Furthermore, the database will facilitate real-world data analysis in precision oncology. For example, prior to the recent FDA-approval of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors for prostate cancer, 50 veterans already had been treated with one of these agents. These data can help further inform which of the many variants of DNA damage repair genes benefit from PARP inhibitors.
Ensuring Access to Care for All Veterans
With any new medical technology comes an obligation to ensure appropriate equal access so as to not exacerbate health care disparities. Veterans enrolled in VA health care are much more likely to live in rural communities than does the US population as a whole, and there was concern that these veterans would not receive NGS testing at the same rate as urban veterans. NPOP therefore was intentional during implementation to ensure rural veterans were being offered testing. Indeed, there has been nearly equal utilization by rurality. No other disparities in NPOP utilization have been seen.
A majority of veterans who undergo testing in NPOP have at least 1 actionable gene variant reported.5 However, some of these are for lower LoE off-label use of FDA-approved medications, but many are for agents available only through clinical trials. Consideration of treatments available through a clinical trial is part of standard practice for patients with advanced malignancies. NPOP data have helped identify cohorts who are eligible for clinical trials on the basis of their tumor DNA sequencing results. The National Oncology Program Office has been working closely with the VA Office of Research and Development to expand access to cancer clinical trials in VA. Veterans also can be treated on trials outside VA as medically appropriate and with local VA approval.
Conclusions
The VA NPOP is one of the largest clinical DNA sequencing programs in the nation with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cuttingedge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
Acknowledgments
NPOP was made possible and implemented through the efforts of a number of people in VHA, including the national and regional leaders who supported the program’s vision and implementation, especially Michael Mayo-Smith, David Shulkin, Jennifer S. Lee, and Laurence Meyer, the leaders and staff of the Massachusetts Veterans Epidemiology Research and Information Center who piloted regional NGS testing, and especially my current and former colleagues in the VA National Oncology Program Office, without whom NPOP would not be possible. The contributions of Neil L. Spector who served as inaugural Director of Precision Oncology and Jill E. Duffy in her role as Director of Oncology Operations are particularly noteworthy.