VA National Precision Oncology Program
Background: The US Department of Veterans Affairs (VA) is the largest integrated health care system in the US. The VA sees about 50,000 new cancer diagnoses each year and provides care for more than 400,000 veterans with cancer. The heterogeneity of molecular testing practice patterns and methods of testing at VA health care facilities and the increasing number and complexity of molecular tests led to the development of national program for precision oncology.
Observations: The National Precision Oncology Program (NPOP) provides tumor sequencing and consultative services for the treatment of veterans with cancer. NPOP is used by nearly all VA oncology practices and has now sequenced > 13,000 samples. The initial focus was on advanced-stage nonsquamous non-small cell lung cancer, which has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, testing was expanded to include metastatic prostate cancer and hematologic malignancies with prior approval. The program also offers cell-free DNA (liquid biopsy) and PD-L1 immunohistochemistry analyses.
Conclusions: The VA NPOP is one of the largest clinical DNA sequencing programs in the US with integrated consultation services and health informatics resources to facilitate patient care, clinical trials, and health outcomes research. The clinical services of NPOP provide cutting-edge oncology services to veterans throughout VA without exacerbating disparities and will be a national resource for research.
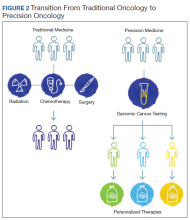
The paradigm of precision oncology, in contrast, utilizes unique, patient-specific molecular characteristics to guide prescribing of antineoplastic agents (Figure 2). These molecular characteristics are frequently tumoral but also may be nontumoral, such as germline genetic variants and even nonhuman, such as the gut microbiome as has been proposed as predictive of response to immune checkpoint inhibitors.1,2
One of the first examples of precision oncology was tumor testing for the estrogen receptor in breast cancer, which distinguishes breast tumors sensitive to hormonal treatments from those that are resistant.3 In 2004, somatically acquired mutation of the EGFR gene was found to be associated with response to EGFR tyrosine kinase inhibitors such as gefitinib and erlotinib, and subsequently it was shown that patients without these mutations derived no benefit from use of these drugs.4 Thus, the precision oncology paradigm is using a molecular diagnostic as part of the indication for an antineoplastic agent, resulting in improved therapeutic efficacy and often reduced toxicity.
By 2015, multiple examples of DNA-based gene alterations that predict drug response were known, including at least 5 in non-small cell lung cancer (NSCLC). The heterogeneity of molecular testing practice patterns and methods of testing in VA along with the increasing number and complexity of molecular tests facilitated launch of a regional precision oncology program based primarily in Veterans Integrated Service Network 1, which provided tumor DNA sequencing through 2 vendors. Advances in DNA sequencing technology, particularly NGS, permit sequencing of multiple genes in clinical tumor samples, using a panel applicable for multiple tumor types. As part of VA contributions to the 2016 White House Cancer Moonshot initiative, the regional program became NPOP with expanded geographic scope, the addition of clinical consultative services, and robust informatics that supports associated research and a learning health care system. NPOP is a component of the VA National Oncology Program Office under the Office of Specialty Care.
Testing
With the launch of NPOP in mid-2016, there was rapid expansion of the number of VA facilities participating, and the number of tumor samples being submitted increased substantially. 5 The expansion was facilitated by both central funding for the tumor DNA sequencing and by NPOP-provided training of pathology laboratory staff and oncologists. Today, NPOP is utilized by almost every oncology practice in VA.
NPOP’s initial focus was on lung cancer, specifically advanced-stage nonsquamous NSCLC, which not only is very common in VA, but also has one of the highest number of mutated genes that result in sensitivity to antineoplastic drugs. Recently, metastatic prostate cancer was added as a second focus tumor type. Dashboards are available on the NPOP website to assist care teams in identifying veterans at their facility with either lung or prostate cancer who may be appropriate for testing. Other solid tumors can be sent for testing through NPOP if patients have advanced stage cancer and are medically appropriate for antineoplastic therapy. To date, NPOP has sequenced > 13,000 samples.
Testing options have been added to NPOP in addition to tumor DNA sequencing. The first addition was the so-called liquid biopsy, more properly known as the cell-free DNA (cfDNA) test, a plasma-based high-sensitivity DNA sequencing assay. cfDNA is shed from dying cells and can be captured and sequenced from a plasma sample obtained by standard venipuncture, using a special-purpose sample collection tube. The test is appropriate for patients who do not have an appropriate archival tumor sample or those who cannot have a new biopsy of tumor tissue. Tumor tissue remains the preferred test sample due to a higher sensitivity than that of cfDNA and less susceptibility to false positives, so consideration of a tumor biopsy is appropriate prior to requesting a cfDNA assay. Therapy can greatly impact the sensitivity of cfDNA testing, so patients should be having disease progression at the time of obtaining a blood sample for cfDNA.