Hardware for the Heart: The Increasing Impact of Pacemakers, ICDs, and LVADs
Evaluating and treating patients with an automated implantable cardiac device requires both an understanding of the components and function of each device, as well as the associated complications.
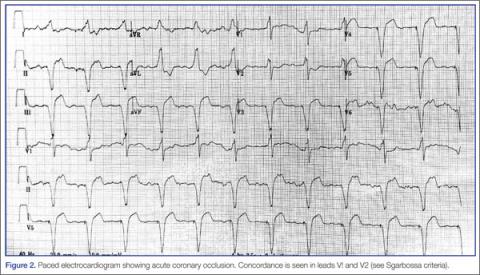
- ST elevation ≥1 mm in a lead with upward (concordant) QRS complex; 5 points.
- ST depression ≥1 mm in lead V1, V2, or V3; 3 points.
- ST elevation ≥5 mm in a lead with downward (discordant) QRS complex; 2 points.13,15
An ECG demonstrating three points of Sgarbossa criteria yields a diagnosis of ST segment elevation MI with 98% specificity and 20% sensitivity.16 A modified Sgarbossa criteria replaces the absolute ST-elevation measurement (Sgarbossa criteria 3) with an ST/S ratio greater than -0.25. This yields a sensitivity of 90% and specificity of 90%.17
Pacemaker-Related Complications
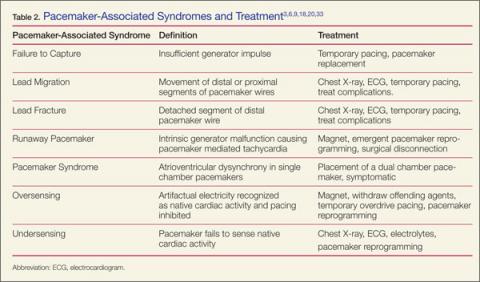
When ischemia is no longer a concern, address the device itself. Workup involves history and physical examination, with complete blood count, chest X-ray, cardiac biomarkers, basic metabolic panel, ultrasound, and device interrogation, as indicated. Table 2 provides a summary of associated pacemaker syndromes and treatment.
Infectious Complications
Patients with device-related infection can present with local or systemic signs, depending on time from implantation. Tenderness to palpation over the generator is sensitive for pocket infection. Although rare, pocket infections require urgent evaluation with mortality rates as high as 20%.18
Early (< 30 days) pocket complications are usually attributable to hematomas with or without infection. When infection is present, Staphylococcus aureus and Staphylococcus epidermidis are the most likely culprits. Up to 50% of isolates can be methicillin resistant S aureus.19 Although needle aspiration has been used in the past for evacuation and microbial identification, current recommendations do not advocate this approach.20 Incision and debridement are the mainstays of therapy. Over 70% of patients with pocket infections will have positive blood cultures and should receive antibiotic therapy with vancomycin.21
Patients with wound separation or pocket infection are at risk for lead infection, lead separation, and lead fracture with related thoracic involvement (ie, pneumonia, empyema, hemothorax, pneumothorax, or diaphragmatic rupture).20
Infectious complications greater than 30 days from implantation are more likely lead-related. Because of the risk for embolic disease to pulmonary or cardiac tissues, emergent line removal and empiric antibiotics are recommended.18 After admission, a transesophageal echocardiogram should be performed to evaluate for valvular involvement and baseline cardiac function.22-24
Physiologic Complications
Patients without ischemia or infection should be evaluated for device-related chest pain. Pain resulting from malfunction of the device usually occurs in the first 48 hours after implantation.9
Patients may present with chest pain related to lead migration or malposition. Perforation of the pleural cavity during the initial procedure can cause hemothorax or pneumothorax. Perforation of the myocardium can lead to hemopericardium and cardiac tamponade. Patients present with respiratory distress and cardiac dysfunction with or without pacing failure.4,9 Bedside cardiac ultrasound assists in assessing these complications and degree of severity.25
Lead migration occurs when a lead detaches from the generator and migrates. Complete separation from the generator may present as failure to capture and should be addressed before lead localization, as temporary pacing may be warranted. Leads coil and regress from patient tampering (ie, Twiddler’s Syndrome) or through spontaneous detachment.3
The ECG may detect functional leads that have migrated to the left heart (coronary sinus, entricular septal defect, perforation). Right bundle branch morphology, rather than the expected left bundle branch morphology, indicates a lead depolarizing the left ventricle.26,27
Lead fracture may occur at any time after implantation. In addition to the complications seen with lead separation and migration, lead fracture is associated with pulmonary vein thrombosis. Because of the volatile nature of fractured leads, patients present more frequently with pacemaker failure, dysrhythmias, and hemodynamic compromise. Temporary pacing may be necessary pending surgical intervention.4,20
Days to weeks postprocedure, patients are at risk for central venous thrombus due to creation of a thrombogenic environment. These thrombi can embolize to the pulmonary circulation and computed tomography pulmonary angiogram should be considered if suspicious.3
Electrical Complications
Failure to pace can be attributed to lead complication (ie, lead malposition, lead fracture), poor lead-tissue interface, or generator complication.28 Electrical complications arise from intrinsic generator malfunction, lack of pacemaker capture, oversensing/undersensing, and poor pacemaker output.29 Poor output results from battery failure, generator failure, or lead misplacement.9
Generator malfunction can produce unwanted tachycardia and exacerbate intrinsic poor cardiac function. Pacemaker-mediated tachycardia (PMT), pacemaker syndrome, and runaway pacemaker should be eliminated from the differential though interrogation and ECG.
Patients presenting with signs of hypotension and cardiac failure may have pacemaker syndrome. With single-chamber conduction, atrioventricular dysynchrony occurs, producing a lack of ventricular preload and poor cardiac output. Treatment includes symptomatic management and pacemaker replacement with a dual-chamber device. In the hemodynamically unstable patient, applications to increase the preload and reduce the afterload should be attempted.20,25