Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
PRACTICE POINT
- For patients with HIV who require systemic therapy for psoriasis, apremilast may provide an effective and safe therapeutic option, with minimal immunosuppressive adverse effects.
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.
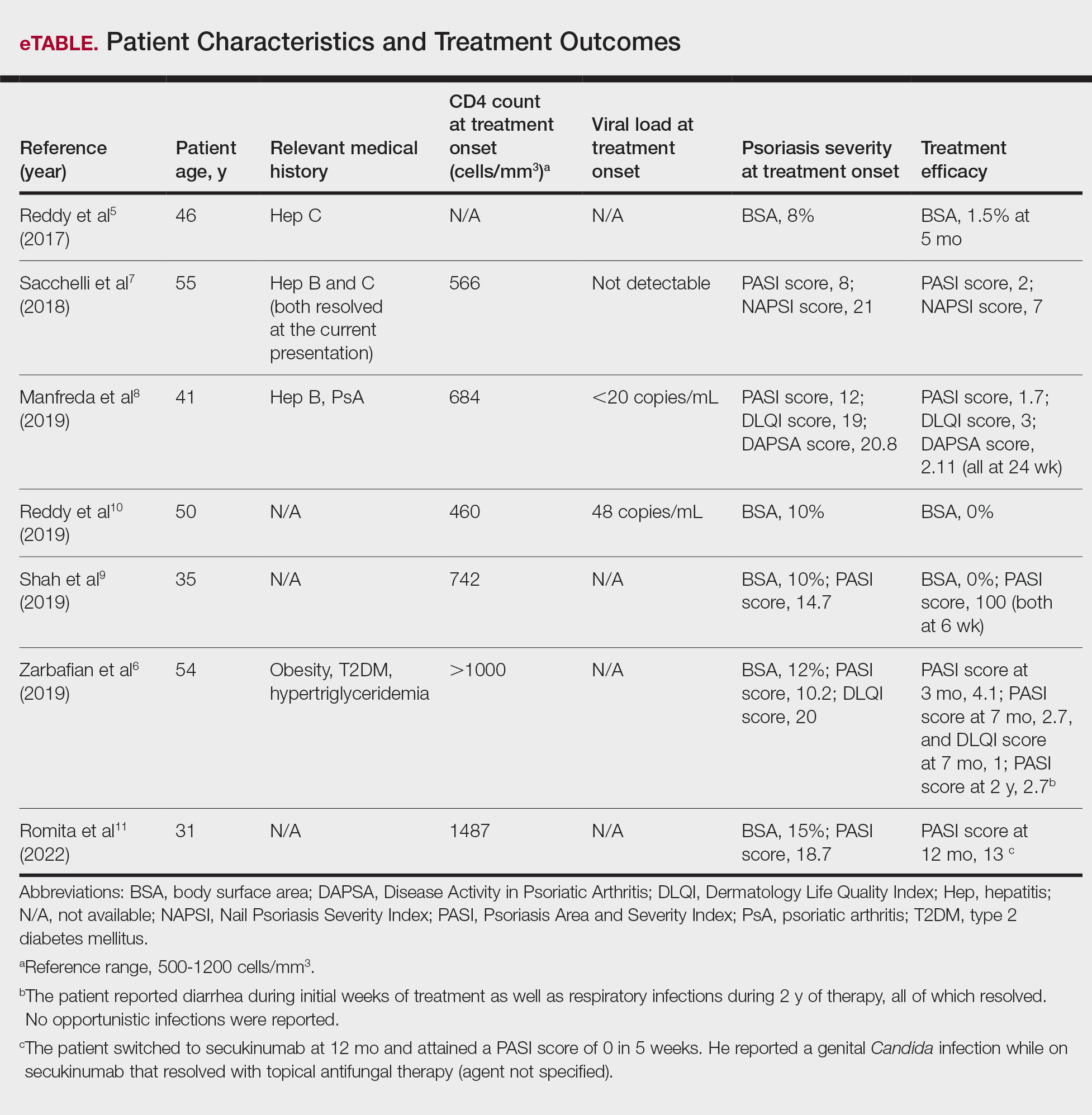
The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.




