Human papillomavirus vaccine: Safe, effective, underused
ABSTRACTVaccination against human papillomavirus (HPV) is safe and effective. It is recommended for females age 9 to 26 and for males age 11 to 26, yet vaccination rates are low. We review the host immune response, the data behind the recommendations for HPV vaccination, and the challenges of implementing the vaccination program.
KEY POINTS
- Two HPV vaccines are available: a quadrivalent vaccine against HVP types 6, 11, 16, and 18, and a bivalent vaccine against types 16 and 18.
- HPV causes cervical cancer, genital warts, oropharyngeal cancer, anal cancer, and recurrent respiratory papillomatosis, creating a considerable economic and health burden.
- The host immune response to natural HPV infection is slow and weak. In contrast, HPV vaccine induces a strong and long-lasting immune response.
- The HPV vaccines have greater than 90% efficacy in preventing cervical dysplasia and genital warts that are caused by the HPV types the vaccine contains. They are as safe as other common prophylactic vaccines.
- HPV vaccination has been challenged by public controversy over the vaccine’s safety, teenage sexuality, mandatory legislation, and the cost of the vaccine.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
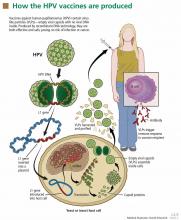
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35